Electrolysis
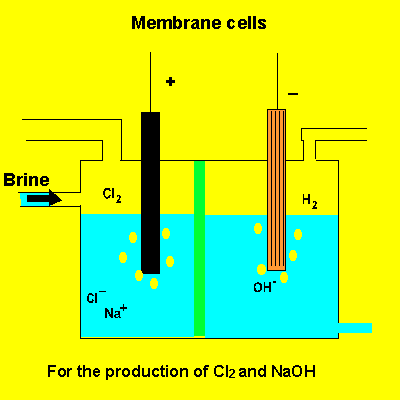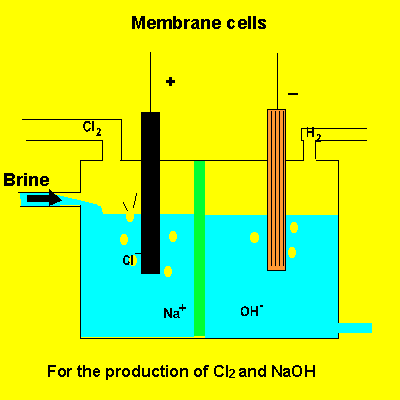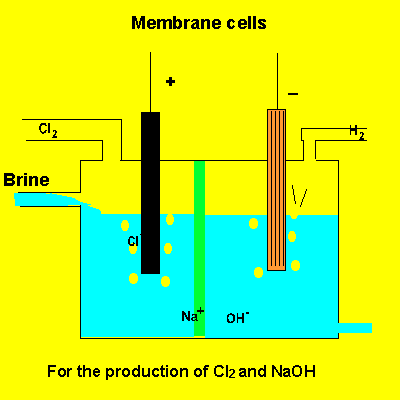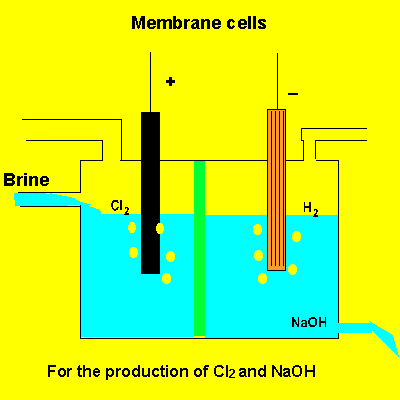
the membrane cell
A key feature of this electrolytic cell is the membrane that separates the products formed at each electrode. Without the membrane present the products would react with each other. Sodium ions can move across the barrier. Chloride ions can not move from one electrode chamber into the next and contaminate the sodium hydroxide formed at the cathode(-).
Sodium chloride(brine) is fed into the reaction chamber continuously. The NaOH is removed and water allowed to evaporate. NaCl, which is present as a contaminant, is less soluble than NaOH and crystalises out. The remaining solution is almost pure NaOH.