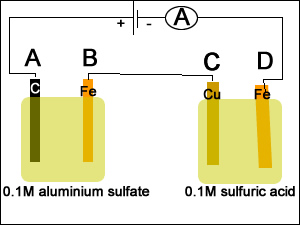
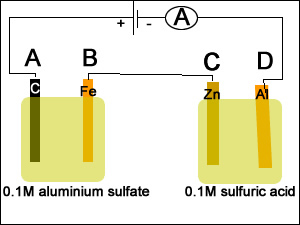
1) A spoon was placed in a silver plating cell for 40 minutes with a current of 30.3A. Calculate the mass of silver deposited on the spoon.
Step 1 Calculate the charge passed through the cell during the 15 minute
period.
Q = I X
t
Q = 30.3 X (40 X 60)s = 72,720C
(time must be expressed in seconds)
Step 2 Calculate
the mole of electrons passed through the circuit.
mole = 72,720C/96,500C/mol
= 0.754 mole.
Step 3 Calculate
the mass of silver
![]()
According to the equation above
0.754 mole of electrons will produce 0.754 mole of silver.
The mass of silver is therefore 0.754 X 107.9 = 81.31grams.
Hide the solution