Electrolysis with the Downs cell

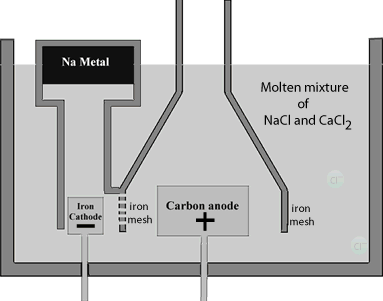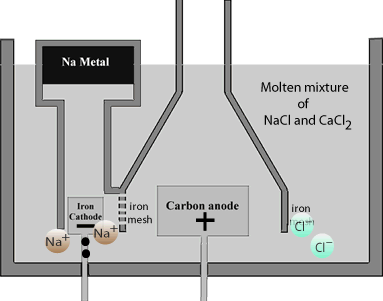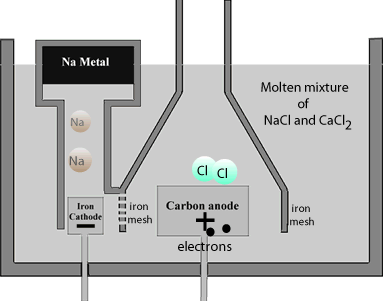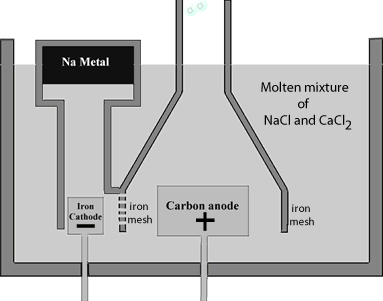
The Downs cell is used in industry to produce sodium metal. The cell consists of a central carbon anode surrounded by a cylindrical iron cathode. An iron mesh screen is used to prevent chlorine gas, formed at the anode, from coming into contact with sodium metal formed at the cathode.
The sodium metal rises in the molten mixture and is collected in a water tight storage vessel. The high current passing through the cell provides sufficient heat to keep the mixture molten. Now sodium chloride melts at approximately 800°C this requires a significant amount of energy to be supplied. Not only is the issue of viability important but at this temperature molten sodium forms a thick fog inside the reaction vessel which is difficult to recover. To lower the melting temperature, calcium chloride is added to the sodium chloride in a 1:2(NaCl:CaCl2) part mixture. The mixture has a much lower melting temperature, around 600°C which prevents the formation of the sodium fog.
The reaction that occurs at the carbon anode is
2Cl-(l) => Cl2(g) + 2e
On the cathode, the iron mesh, electrons are forced onto the most reactive sodium cations, the reaction is as follows
Na+(l) + e=> Na(l)