Advanced exercises
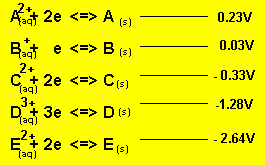
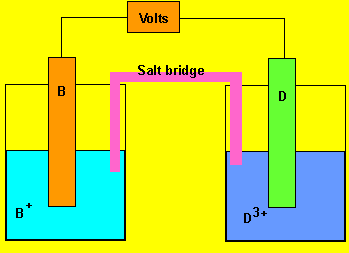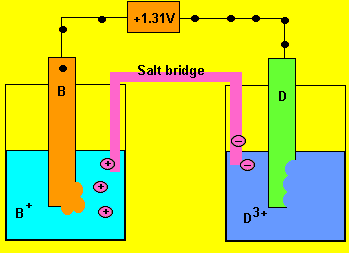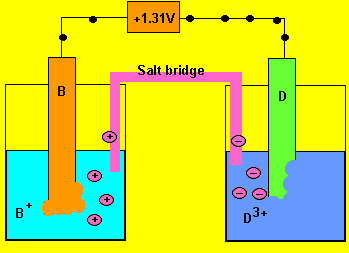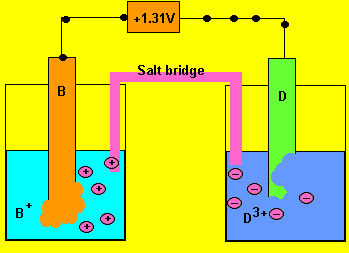
Using the reactivity series above, answer the following questions for each cell.
a) What is the potential
difference generated by the cell?
Cell emf = 0.03 - - 1.28 = 1.31V
b) In which direction do the electrons flow?
From electrode "D" to "B"
c) What happens to the mass of each electrode ?
Electrode "D" decreases while "B" increases.
d) Which of the two electrodes is the anode and which is the cathode?
Oxidation always occurs at the anode so electrode "D"
is the anode (-) and "B" is the cathode(+)
e) What charged particles flow into each half cell?
Positive ions flow into the half cell on the left to overcome
the negative charge building up. Negative ions flow into the cell on
the right.
f) Give the overall redox reaction