· Set up a table similar to the one shown below.
| Chemical |
Silver nitrate |
Lead |
Copper sulfate |
Potassium iodide | Sodium carbonate | Sodium chloride | Potassium chromate | Barium chloride |
| Silver nitrate |
||||||||
| Lead nitrate |
||||||||
| Copper sulfate |
||||||||
| Potassium iodide | Yellow precipitate | |||||||
| Sodium carbonate | ||||||||
| Sodium chloride | ||||||||
| Potassium chromate | ||||||||
| Barium chloride |
· Mix the solutions as per table in a micro-test tube.
· Observe any solid that may be formed.
· If a precipitation reaction does occur name the precipitate and describe its appearance.
Naming the precipitate
- Simply swap the first names of the two solutions.
Eg. When lead nitrate and potassium iodide solutions are mixed.
Swapping the first names we come up with the names
Lead iodide
Potassium iodide.
Now keep in mind that if the name of a compound contains the words, potassium, nitrate, sodium or ammonium it can not be a precipitate as these compounds are always soluble and precipitates need to be insoluble.
What is the name of the precipitate formed when silver nitrate solution is mixed with sodium chloride solution?
If quantities of solutions is scarce than use a black tile and place drops of each solution in a grid as indicated by the table.

|
Carbonate
ions |
Chloride
ions |
Sulfide
ions |
|
|
Barium |
Precipitate
formed |
||
|
Cadmium |
Precipitate
formed |
Precipitate
formed |
|
|
Silver |
Precipitate
formed |
Precipitate
formed |
Precipitate
formed |
The student has a solution of dissolved barium, cadmium and silver. She wishes to separate solid compounds of each metal from the solution by filtering the precipitates formed.
In what order must she add the solutions of carbonate, chloride and sulfide so that she filters out a compound of each metal in turn?
A chemical contractor is suspected of pouring dangerous
chemicals into the river system and polluting the local water supply.
Heavy metals appeared in the water and caused serious health problems
in the local community. In trying to identify the metals present, a study
was undertaken. Samples of water were collected
and tested with the following chemicals.
Potassium iodide gave a yellow precipitate. What metals could be present?
Explain
A sample of water is suspected of having lead or silver, but not both. Describe a series of reactions that can be used to identify the metal.

A sample of storm water, from an industrial estate, was tested with four solutions. The results for three of those solutions is shown on the right. Testing with lead nitrate solution did not produce a precipitate. Using the results of your experiment identify the likely contaminant found in the storm water.
Click on the picture to enlarge it.
Silver iodide is a precipitate that can be formed between
The crystal structure of AgI is similar to that of ice, allowing it to induce freezing of water in clouds . Silver iodide is used in cloud seeding.
What property of silver iodide makes it useful in photography?

White precipitates are very common. A sample of water from a nearby river was tested with a chloride solution. A white precipitate is formed. The locals suspect that lead is present. Suggest further tests that can be carried out and what results can be expected to show the presence or absence of lead.
A Jewelery store was robbed of silver valued at one million
dollars. The silver was dissolved by chemical means and sold. A suspect
was arrested and his clothing tested. Describe two tests that can be conducted
to see if silver metal is present on the suspect's clothing.
Another clever use of precipitation reactions was during WWII to camouflage submarines. Click to read.
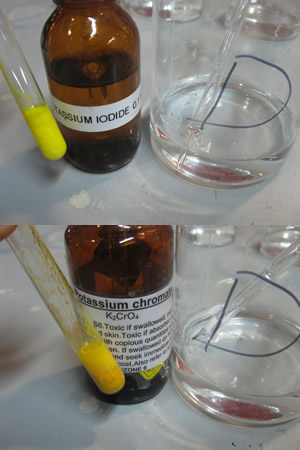
The picture on the right shows a red precipitate formed when silver nitrate is mixed with potassium chromate.
What is the name of the precipitate in the filter paper?
Explain why it is not a pure sample.
How can we obtain a pure sample of this precipitate?

