Periodic table and the reactive metals
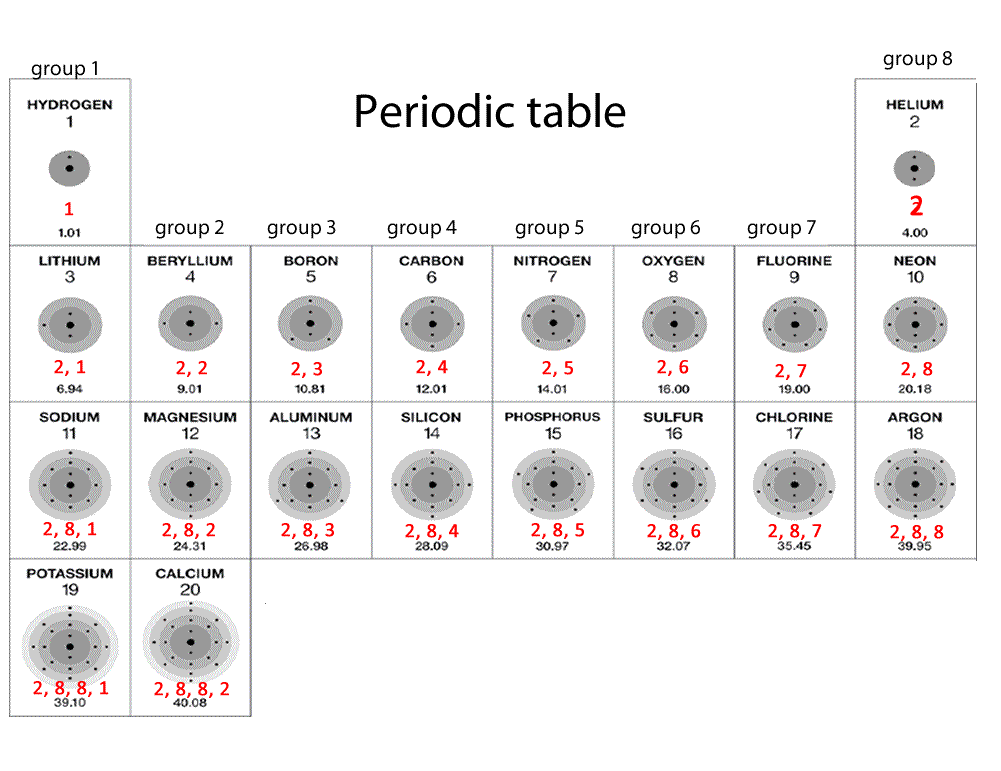
The periodic table is a way of organizing known elements into groups with
similar properties. Mendelev was a Russian scientist who was first to
recognise that elements can be grouped together according to atomic mass. All elements in a particular group share similar chemical properties. All elements in group 1 are very reactive metals, with the exception of hydrogen gas. These metals react readily with other compounds and elements to give away some of their outer shell (valence) electrons. These metals react with water to produce hydrogen gas. Click on the blue writing below to see the reactions.
|
|
| Sodium reacts slowly with water to produce hydrogen gas. Hydrogen gas builds up and ignites. | |
Potassium reacts faster than sodium to produce hydrogen gas. |
|
| Cesium reacts more violently with water |
|
Elements in group 2 also react with water to form hydrogen gas but are not as reactive as group 1 elements. Magnesium |
|
| Calcium |
|
Notice how reactivity of metals increases as we go down a group. Watch the videos above and compare the reactivity of sodium and potassium with water. Now compare the reactivity of calcium and magnesium with water. Is there a pattern? |
|