1) The thermochemical equation for the complete combustion of glucose is
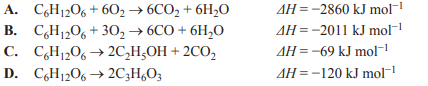
Solution

2) A reaction has the energy profile diagram shown below.
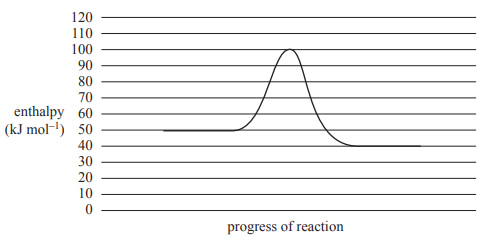 Which of the following represents the energy profile of the reverse reaction?
Which of the following represents the energy profile of the reverse reaction?
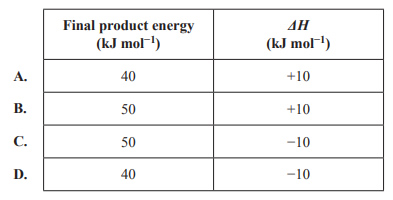
Solution

3) Which one of the following statements about enthalpy change is correct?
A. The sign of the enthalpy change for an endothermic reaction is negative.
B. The sign of the enthalpy change for the condensation of a gas to a liquid is negative.
C. The enthalpy change is the difference between the activation energy and the energy of the reactants.
D. The enthalpy change is the difference between the activation energy and the energy of the products.
Solution

4) Internal combustion engines are used in large numbers of motor vehicles. Historically, internal combustion engines have used fuels obtained from crude oil as a source of power. As concerns for the environment have grown, efforts have been made to obtain fuel for combustion engines from other sources.
a. One way of reducing the environmental effects of fossil fuels is to blend them with biofuels. A common method is to blend petrol with ethanol in varying ratios. A fuel can be obtained by blending 1 mole of octane, C8H18, and 1 mole of ethanol, C2H5OH. The chemical equation for the complete combustion of this fuel mixture is
![]()
Calculate the energy released, in kilojoules, when 80 g of this fuel mixture undergoes complete combustion. Show your working
Solution

6) There are many varieties of bread available to consumers in Australia. The nutritional values for one type of wholemeal bread are given in the table below.
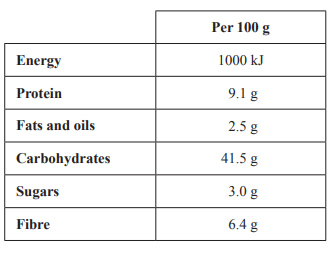
The wholemeal bread undergoes complete combustion in a bomb calorimeter containing 200 g of water. Assume that all of the energy in the combustion is transferred to the water.
i. Calculate the mass of bread needed to raise the temperature of the water by 6 °C.
Solution

ii. The combustion of the bread was investigated using a different method. The bread was ignited under a beaker containing 200 g of water, which was set on a tripod. The equipment used is shown below.
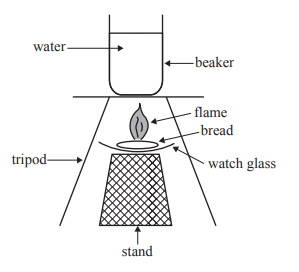
If 1.2 g of bread was needed to raise the temperature of the water by
6 °C using this different method, calculate the efficiency of the energy transfer in this combustion.
Solution
