1) A clear, colourless liquid extract of the rhubarb plant was analysed for the concentration of oxalic acid, H2C2O4, by direct titration with a recently standardised and acidified potassium permanganate solution, KMnO4(aq). The balanced equation for this titration is shown below.
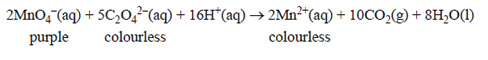
The steps in the titration were as follows:
Step 1 – A 20.00 mL aliquot of the rhubarb extract was placed in a 200 mL conical flask.
Step 2 – The burette was filled with acidified 0.0200 M KMnO4 solution.
Step 3 – The acidified 0.0200 M KMnO4 solution was titrated into the rhubarb extract in the conical flask. The titration was considered to have reached the end point when the solution in the conical flask showed a permanent change in colour to pink. The volume of the titre was recorded.
Step 4 – The titration was repeated until three concordant results were obtained. The average of the concordant titres was 21.7 mL.
The concentration of H2C2O4 in the rhubarb extract is closest to
A. 5.43 × 10–2 M
B. 5.00 × 10–2 M
C. 2.17 × 10–2 M
D. 7.40 × 10–2 M
Solution

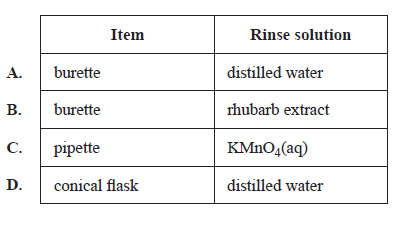
2) Which of the following rinses is least likely to affect the accuracy of the results?
Solution
