1) The following two spectra were obtained for a pure organic substance, Compound W.
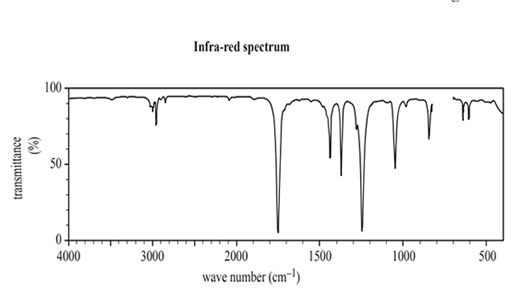
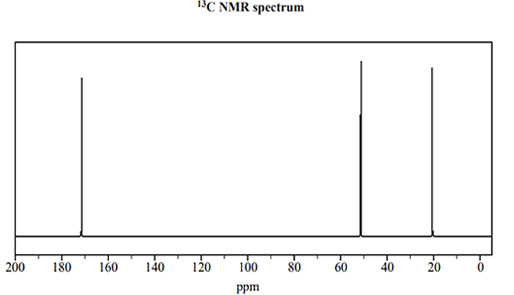
The formula of Compound W that is consistent with the spectra above is
A. CH2(OH)CH2CH2OH
B. CH3CH2COOH
C. CH3COOCH3
D. CH3COCH3
Solution

2) The mass spectrum shown above is for a molecule with the molecular formula C4H7Cl. Which species is responsible for the base peak?
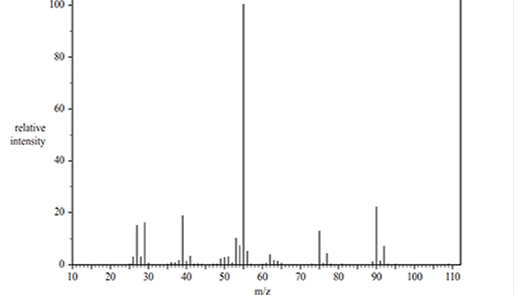

Solution

3) A student investigated an organic substance, Compound Y, with the molecular formula C5H8O
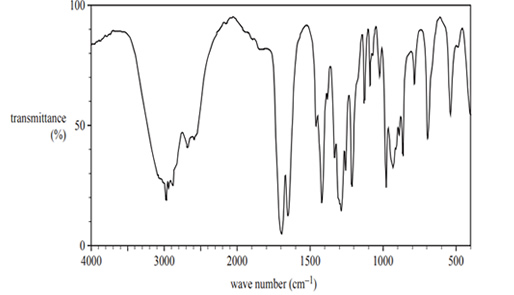
The infra-red spectrum of Compound Y is shown above. On the spectrum, circle two peaks and identify the bond(s) responsible for each peak.
Solution

4) A sample of Compound Y was further analysed using 13C NMR and
1H NMR. The spectra are shown below.
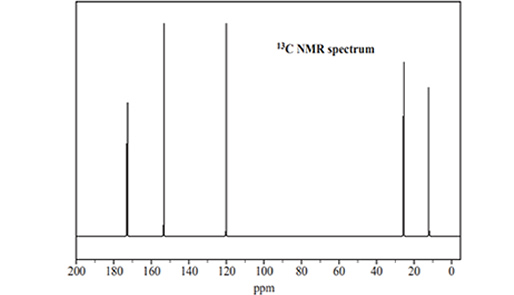
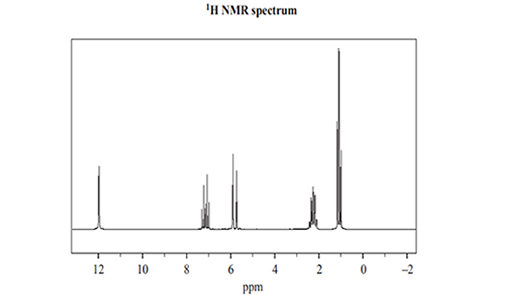
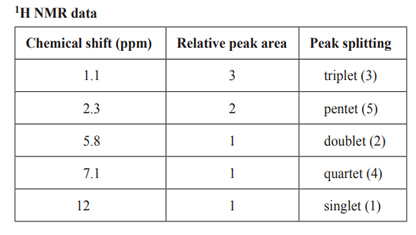
a) Use the information provided in the 13C NMR spectrum to identify the number of different carbon environments for Compound Y.
b) For the signal at 2.3 ppm in the 1H NMR spectrum, identify what specific information is provided by
• the relative peak area
• peak splitting
d) Draw the structural formula of Compound Y.
Solution
