1) Shown below is the infra-red spectrum of an organic compound.
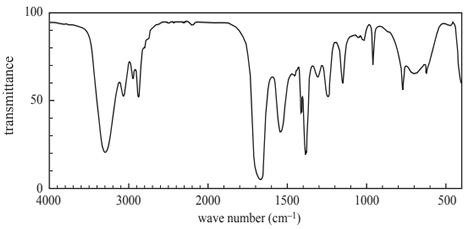
The organic compound that produces this spectrum is an
A. aldehyde.
B. alcohol.
C. amide.
D. ester
Solution

2) There are a number of structural isomers for the molecular formula
C3H6O. Three of these are propanal, propanone and prop-2-en-1-ol. The mass spectrum below was produced by one of the three named isomers of C3H6O.
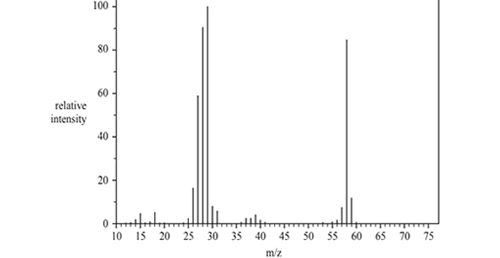
a) Identify the fragment at 29 m/z.
Solution

b) Name the isomer of C3H6O. that produced this spectrum and justify your answer..
Solution

c) Consider the 13C NMR and 1H NMR spectra below
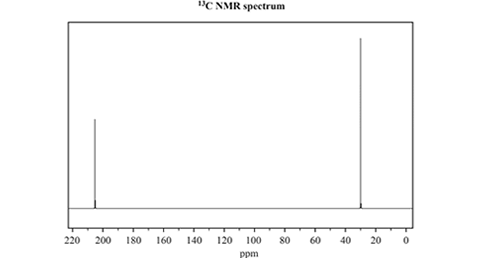
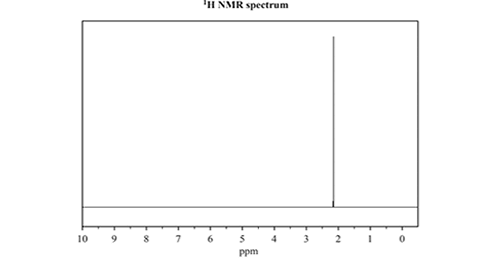
Identify which one of the three named isomers of C3H6O produced the NMR spectra. Justify your answer by referencing both spectra.
Solution
