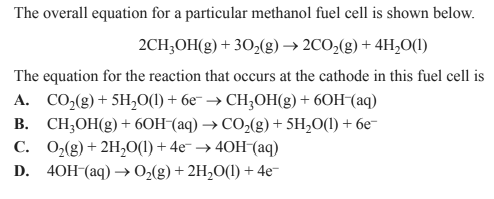
Solution

2)Submarines operate both on the surface and underwater. When operating underwater, the submarine acts as a closed system, where there is no interaction with the atmosphere. Most types of submarines use both batteries and diesel engines to provide their energy requirements. A new type of submarine uses proton exchange membrane (PEM) fuel cells and diesel engines. Below is a diagram of a PEM fuel cell.
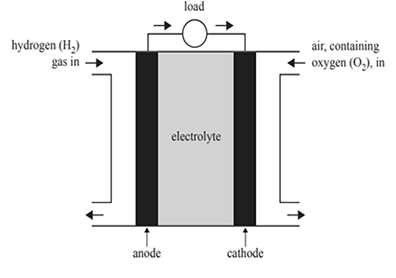
a) State the function of the electrolyte in a fuel cell.
Solution

b) Write the balanced overall redox reaction that occurs in this PEM fuel cell.
Solution

c) Give two safety considerations for the safe storage of hydrogen, H2, gas on a submarine.
Solution

d) State two advantages of using a PEM fuel cell compared to a diesel engine when a submarine is underwater
Solution

e) Most submarines generate more H2 gas for their fuel cells when travelling on the surface. Explain how the H2 gas could be generated.
Solution
