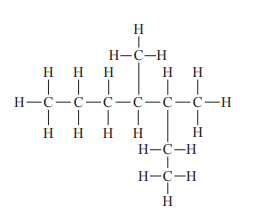
1) What is the correct systematic name for the compound shown above? A. 4-methyl-5-ethylhexane
B. 2-ethyl-3-methylhexane
C. 4,5-dimethylheptane
D. 3,4-dimethylheptane
Solution

2) Met-enkephalin (Tyr–Gly–Gly–Phe–Met) is a peptide found in the central nervous system and the gastrointestinal tract of the human body. Which of the following are the correct structures for the two terminal ends of met-enkephalin at a very low pH?
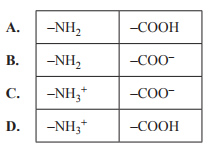
Solution

3) A condensation reaction involving 200 glucose molecules, C6H12O6, results in a polysaccharide. The molar mass, in g mol–1, of the polysaccharide is
A. 36 000
B. 35982
C. 32 418
D. 32 400
Solution

4) The molecule with the structural formula shown below reacts with hydrogen bromide, HBr, to form C5H11Br.
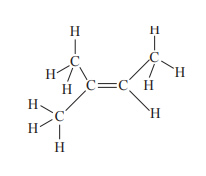
The number of different structural isomers theoretically possible to be produced by this reaction is
A. 1
B. 2
C. 3
D. 4
Solution

5) When ethene is mixed with chlorine in the presence of UV light, the following reaction takes place.

Reactions of organic compounds can be classified in a number of ways. The following list shows four possible classifications:
1. addition
2. substitution
3. redox
4. condensation
Which classification(s) applies to the reaction between ethene and chlorine?
A. 1
B. 1 and 2
C. 1 and 3
D. 4
Solution

6) Substance P is a peptide found in the human body, and it is associated with inflammation and pain. The structure of Substance P is shown below.
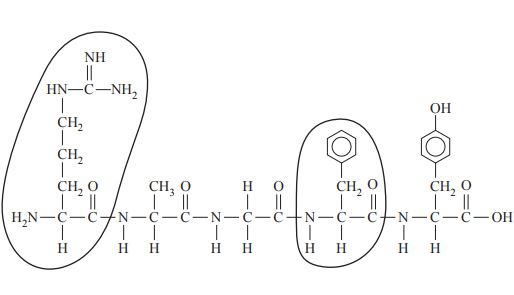
What are the abbreviated names of the two circled amino acid residues? A. Arg and Phe
B. Lys and Tyr
C. Phe and Tyr
D. Met and Arg
Solution

7) Butanoic acid is the simplest carboxylic acid that is also classified as a fatty acid. Butanoic acid may be synthesised as outlined in the following reaction flow chart.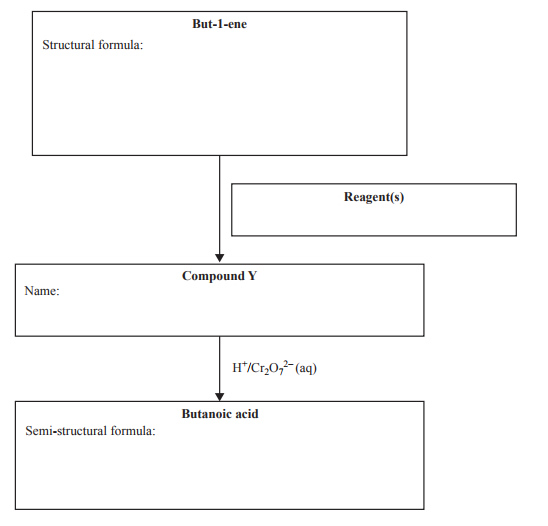
i. Draw the structural formula of but-1-ene in the box provided.
Solution
ii. State the reagent(s) needed to convert but-1-ene to Compound Y in the box provided.
Solution
iii. Write the systematic name of Compound Y in the box provided.
Solution
iv. Write the semi-structural formula of butanoic acid in the box provided.
Solution
v. Write a balanced half-equation for the conversion of Cr2O72– to Cr3+.
Solution

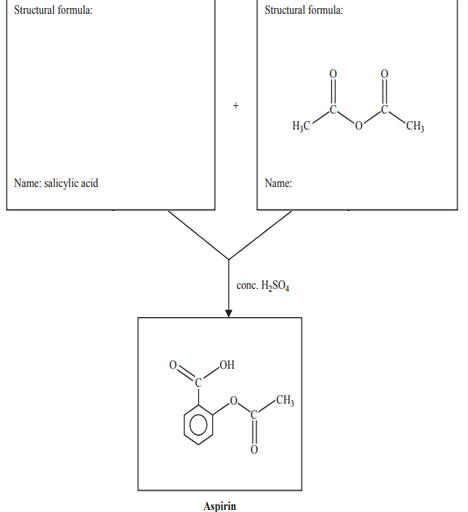
8) An incomplete reaction pathway for the synthesis of aspirin is given above.
i. Draw the structural formula of salicylic acid in the box provided.
Solution
ii. The structural formula of the other reactant is provided. State its systematic name in the box provided
Solution

9) NH2CH2CH2NH2 forms a condensation polymer with butanedioic acid,
HOOCCH2CH2COOH. Draw the structure of the repeating unit on the copolymer that would be formed.
Solution
