1) An electrolytic cell is set up to obtain pure copper from an impure piece of copper called 'blister copper. The electrolyte solution contains both copper(II) sulfate and sulfuric acid. The blister copper, Electrode I, contains
impurities such as zinc, cobalt, silver, gold, nickel and iron. The cell voltage is adjusted so that only copper is deposited on Electrode II. Sludge, which contains some of the solid metal impurities present in the blister copper,
forms beneath Electrode I. The other impurities remain in solution as ions.
The diagram below represents the cell.
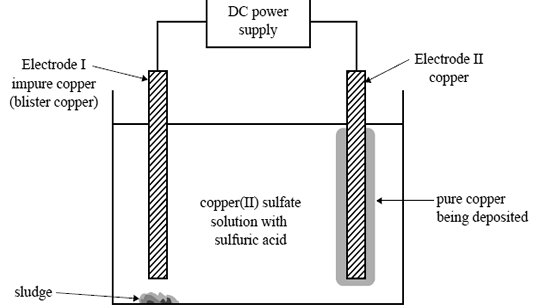
The solid metal impurities that are found in the sludge are
A. gold, nickel and cobalt.
B. cobalt, nickel and iron.
C. nickel and iron.
D. silver and gold.
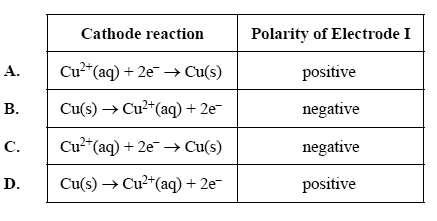
2) Which of the following correctly shows both the equation for the reaction occurring at the cathode and the polarity of Electrode I?
Solution

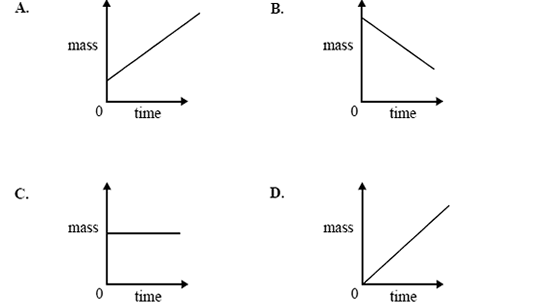
3) Which one of the following graphs best shows the change in mass of Electrode I over a period of time, starting from
the moment the power supply is connected?
Solution
