1) Magnesium is one of the most abundant elements on Earth. It is used extensively in the production of magnesium-aluminium alloys. It is produced by the electrolysis of molten magnesium chloride. A schematic diagram of the electrolytic cell is shown below.
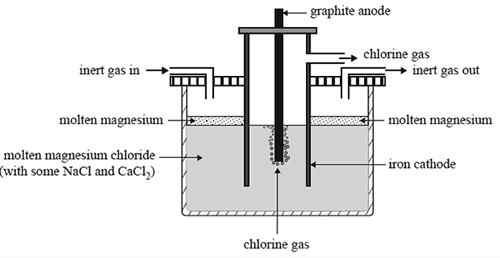
The design of this cell takes into account the following properties of both magnesium metal and magnesium chloride:
• Molten magnesium reacts vigorously with oxygen.
• At the temperature of molten magnesium chloride, magnesium is a liquid.
• Molten magnesium has a lower density than molten magnesium chloride and forms a separate layer on the surface.
a) Write a balanced half-equation for the reaction occurring at each of
• the cathode
• the anode.
Solution
Examiners comments regarding loss of marks by the use of equilibrium arrows and lack of states.

b) Explain why an inert gas is constantly blown through the cathode compartment
Solution

c)The melting point of a compound can often be lowered by the addition of small amounts of other compounds. In an industrial process, this will save energy. In this cell, NaCl and CaCl2 are used to lower the melting point of MgCl2.
Why can NaCl and CaCl2 be used to lower the melting point of MgCl2 but ZnCl2 cannot be used?
d) What difference would it make to the half-cell reactions if the graphite anode were replaced with an iron anode? Write the half-equation for any different half-cell reaction. Justify your answer.