1) Chemists suspected that an impure copper lump contained a signifi cant amount of cobalt. Cobalt would be
oxidised to Co2+ ions that would remain in the electrolyte solution. The spectrogram below gives the results
of analysis of the solution. The two ions absorb at distinctly different wavelengths.
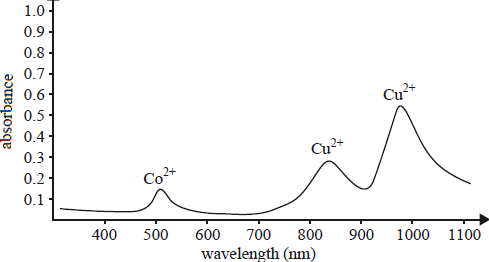
a) i)Which analytical technique was used to perform this analysis?
A calibration graph was constructed using Co2+(aq) solutions of known concentrations.
ii. What wavelength would you select to construct this curve?
Solution

b) A Co2+(aq) solution of unknown concentration registered an absorbance reading of 0.350. Determine the concentration of Co2+ ions in this solution.
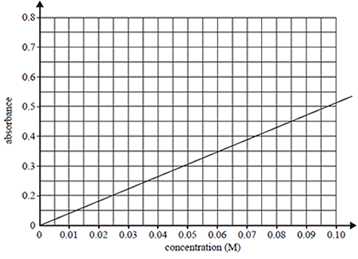
Solution

2) An unknown organic compound, molecular formula C4H8O2, was presented to a spectroscopy laboratory for identification. A mass spectrum, infrared spectrum, and both 1H NMR (proton NMR) and 13C NMR spectra were produced. Click on the blue writing to see the spectra.
The analytical chemist identifi ed the compound as ethyl ethanoate.
A report was submitted to justify the interpretation of the spectra. The chemist's report indicating information about the structure provided by the 13C NMR spectrum has been completed for you.
a. Complete the rest of the report by identifying one piece of information from each spectrum that can be used to identify the compound.
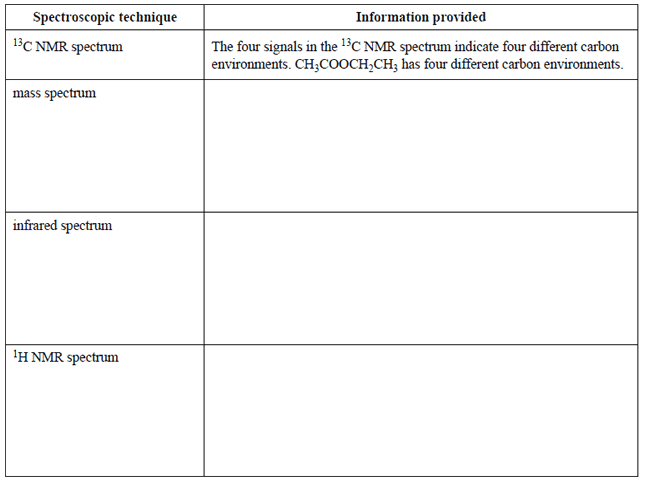

c) Another compound has the same molecular formula as ethyl ethanoate. However, the carbon 13C NMR spectrum of this compound shows only three signals.
Draw a possible structure of this compound.
Solution
