1)
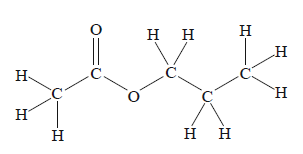
The systematic IUPAC name for the molecule shown above is
A. ethyl ethanoate.
B. ethyl propanoate.
C. propyl ethanoate.
D. methyl propanoate.
Solution

2)
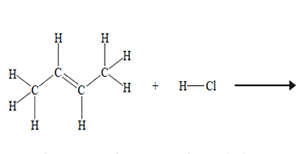
The systematic IUPAC name for the product of the above chemical reaction is
A. 1-chlorobutane.
B. 2-chlorobutane.
C. 3-chlorobutane.
D. 4-chlorobutane.
Solution
3) The reaction pathway for the synthesis of paracetamol, a mild painkiller, is provided below.
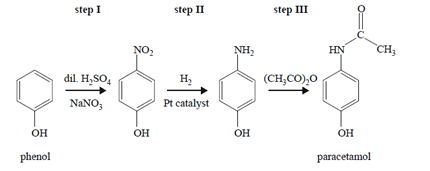
Which step or steps in this synthesis involve(s) a reduction reaction?
A. step I only
B. step II only
C. steps I and III only
D. steps I, II and III
Solution

4) The reaction pathway below represents the synthesis of compound C.
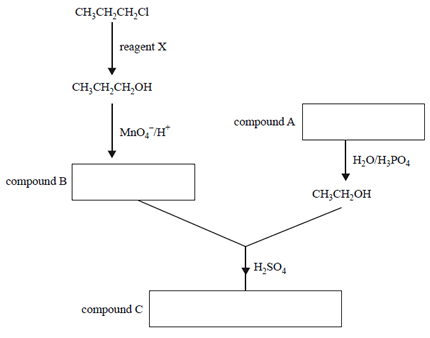
a) Identify reagent X.
Solution

b) In the appropriate boxes above, write the semi-structural formulas for compounds A, B and C.
Solution

c) Give the systematic IUPAC names for compounds A and B.
compound A ______________________
compound B______________________
Solution

5) Olive oil, which has been part of the human diet for thousands of years, is derived from the fruit of the olive tree.
The main fatty acid that makes up olive oil is oleic acid,
CH3(CH2)7CH CH(CH2)7 COOH.
The triglyceride formed from three oleic acid molecules is glycerol trioleate, C57H104O6. The molar mass of
glycerol trioleate is 884 g mol–1.
a. i. An incomplete semi-structural formula of glycerol trioleate is provided below.
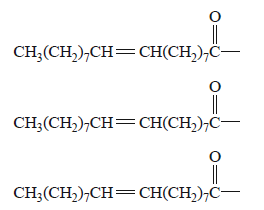
Complete the semi-structural formula of glycerol trioleate.
Solution

b) Explain why oleic acid is described as a mono-unsaturated fatty acid
Solution

6) Ethanol for use as a biofuel can be produced from the fermentation of monosaccharides, such as glucose,
C6H12O6, which is derived from polysaccharides found in plants.
Write an equation for the fermentation reaction of glucose.
Solution
