1) The reaction between hydrogen and oxygen is the basis of energy production in a number of fuel cells
![]()
An alkaline electrolyte is used in a particular hydrogen/oxygen fuel cell.
a) Write a balanced half-equation for the reaction occurring at the
i. cathode
ii. anode.
Solution

2) What is the maximum voltage predicted for one alkaline hydrogen/oxygen fuel cell under standard conditions?
Solution

b) Much of the hydrogen used in fuel cells is produced from methane.
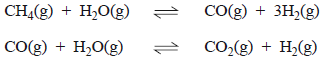
Explain why methane generated by biomass is a renewable fuel while methane derived from fossil fuels is not.