1) A student prepares 1.0 M aqueous solutions of AgNO3, Fe(NO3)2 and KNO3.
Equal volumes of each solution are placed in separate beakers, identical platinum electrodes are placed in each
beaker and each solution undergoes electrolysis with the same current applied for 5.0 minutes under SLC. Each
cathode is then dried and weighed to determine mass change.
Assume that the concentrations of the solutions have decreased only slightly.
In order of increasing mass, the metals deposited on the three cathodes are likely to be
A. potassium, silver, iron.
B. silver, iron, potassium.
C. iron, potassium, silver.
D. potassium, iron, silver.
Solution

2) An electrolytic process known as electrorefining is the final stage in producing highly purifi ed copper. In a
small-scale trial, a lump of impure copper is used as one electrode and a small plate of pure copper is used
as the other electrode. The electrolyte is a mixture of aqueous sulfuric acid and copper sulfate.
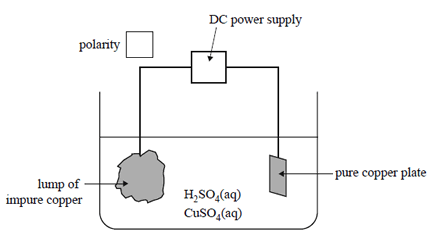
Indicate in the box labelled 'polarity' on the diagram above, the polarity of the impure copper
electrode.
a) Indicate in the box labelled 'polarity' on the diagram above, the polarity of the impure copper electrode.
Solution
In a trial experiment, the electrodes were weighed before and after electrolysis. The results are provided in the following table.

On the basis of these results
• calculate a percentage purity of the lump of impure copper
• indicate one factor that may affect the accuracy of these results.
Solution
Examiner's report includes the statement that students are required to apply their chemical knowledge to unfamiliar situations.

b) Conditions in the electrolytic cell shown in the diagram are carefully controlled to ensure a high degree of copper purity and electrical efficiency. Use the mass of pure copper deposited that is given in the table in part a. to determine the time, in days, taken for this electrolysis reaction to be completed. Assume the current was a constant 24 A.
Solution

c) Lumps of impure copper typically contain impurities such as silver, gold, cobalt, nickel and zinc. Cobalt, nickel and zinc are oxidised from the copper lump and exist as ions in the electrolyte. Silver and gold are not oxidised and form part of an insoluble sludge at the base of the cell.
Why is it important that silver and gold are not present as cations in the electrolyte?
Solution
