Question 1
Students in a chemistry class were required to design a procedure to determine gravimetrically the concentration of lead(II) ethanoate, Pb(CH3COO)2, in a sample of hair dye. They were instructed to measure the mass of precipitate formed when a sample of the hair dye was added to either 0.1 M potassium iodide or 0.1 M potassium nitrate.
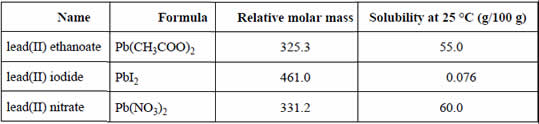
Student A decided to precipitate the lead(II) ions in the hair dye as lead(II) iodide. She added an excess of 0.1 M KI solution to 20.0 mL of hair dye. The yellow precipitate was filtered using pre-weighed filter paper. The precipitate was then washed with distilled water. The precipitate and filter paper were gently heated, allowed to cool and then weighed. This step was repeated several times.
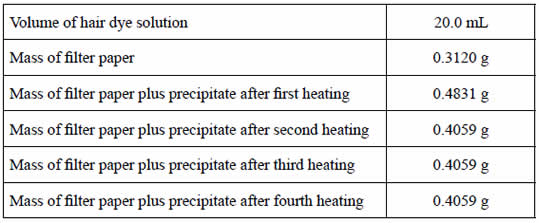
Student A's results are summarised on the right.
a. i. Write a balanced equation for the precipitation of lead(II) iodide.
Solution

ii. Explain why the filter paper and precipitate were heated and weighed several times.
Solution

iii.Calculate the mass, in grams, of lead(II) iodide formed.
Solution

iv. What is the mass, in grams, of lead(II) ethanoate that is present in 100.0 mL of hair dye solution?
Solution

v. Student B decided to precipitate the lead(II) ions in the hair dye as lead(II) nitrate. However, he did not produce any precipitate. Explain why no precipitate of lead(II) nitrate formed.
Solution
