1) A section of DNA is shown on the right in simplified form.
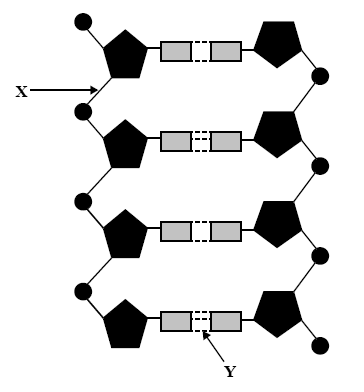
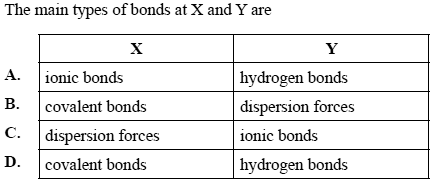
Solution

2) In a double-stranded DNA sample, adenine constitutes 16% of the total number of bases.
The percentage of guanine content in the double strand is
A. 16%
B. 34%
C. 42%
D. 68%
Solution

3) Consider the following statements about the structure of proteins.
I The primary structure of a protein is determined by the sequence of amino acid residues.
II The secondary structure of a protein is the result of hydrogen bonding between –NH and –CO groups.
III The tertiary structure of a protein involves bonding between the side chains on the amino acid residues.
Of these statements
A. only I and III are true.
B. only I and II are true.
C. only II and III are true.
D. I, II and III are all true.
Solution

4) Which one of the following amino acids is likely to be most polar in an aqueous solution at pH 7?
A. glutamic acid
B. glycine
C. leucine
D. valine
Solution

5) Enzymes play an important role in biochemical reactions. Consider the following statements relating to enzyme-catalysed reactions.
I The shapes of the substrate and the active site of the enzyme are complementary.
II When enzymes are denatured, the shape and structure of the active sites are not altered.
III The substrate forms bonds with the active site of the enzyme.
Of these statements
A. only I is true.
B. only III is true.
C. only I and III are true.
D. I, II and III are all true.
Solution

6) Triglycerides are an important source of energy in the body. During digestion, triglycerides are broken down in the small intestine by the enzyme lipase. An incomplete chemical equation that shows the hydrolysis of a triglyceride is shown below.
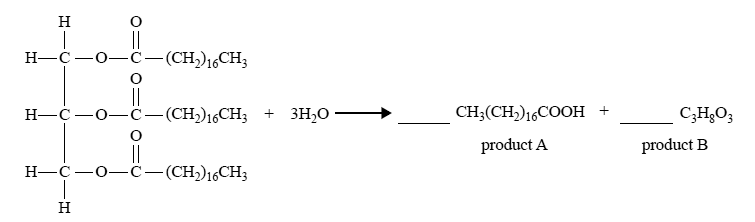
i) In the spaces provided above, balance the equation by adding appropriate coefficients for product A and product B.
Solution

ii) Name the fatty acid that is produced by the hydrolysis of this triglyceride.
Solution
iii) The fatty acid produced in the above reaction is completely oxidised to produce carbon dioxide and water.
Write a balanced equation for the oxidation reaction.
Solution