1) Two different food dye samples, W and Z were compared using thin layer chromatography as shown below.
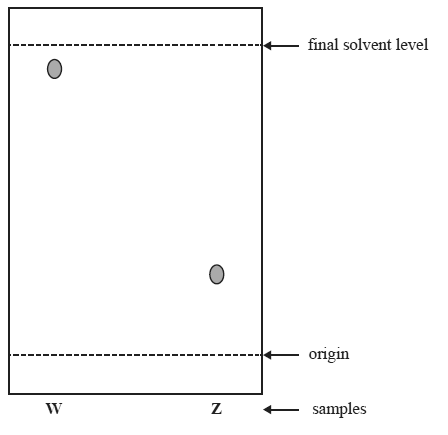
a) Z is more strongly adsorbed than W and has a lower Rf value.
b) Z is more strongly adsorbed than W and has a higher Rf value.
c) W is more strongly adsorbed than Z and has a lower Rf value.
d) W is more strongly adsorbed than Z and has a higher Rf value.
Solution

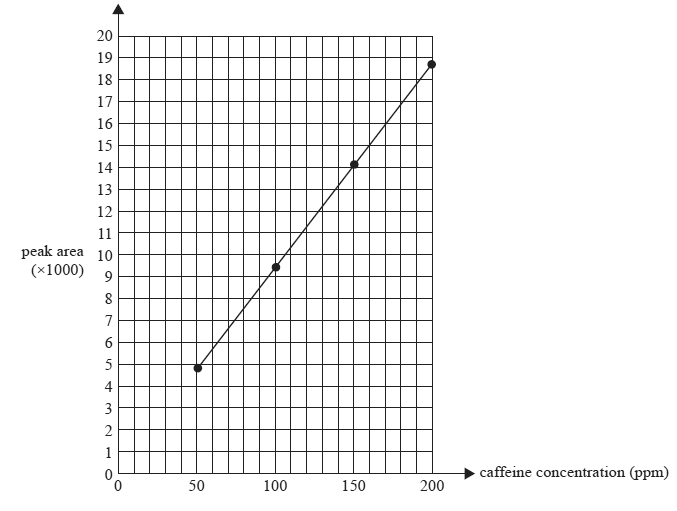
2) Caffeine is a stimulant drug that is found in coffee, tea, energy drinks and some soft drinks. The concentration of caffeine can be determined using HPLC. Four caffeine solutions containing 50 ppm, 100ppm, 150ppm and 200 ppm were prepared. 25 microliters of each sample was injected into the HPLC column. The peak areas were measured and used to construct the calibration graph below. The chromatograms of the standard solution each produced a single peak at a retention time of 96 seconds.
Peak area of caffeine standard solutions: retention time = 96 seconds
25 micro-litre samples of various drinks thought to contain caffeine were
then separately passed through the HPLC column. The results are shown below.
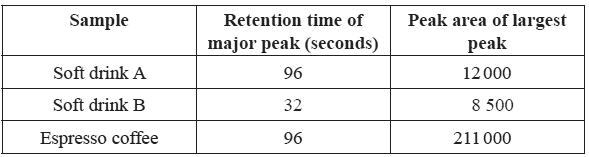
a)Determine the caffeine content in ppm for drink A
Solution

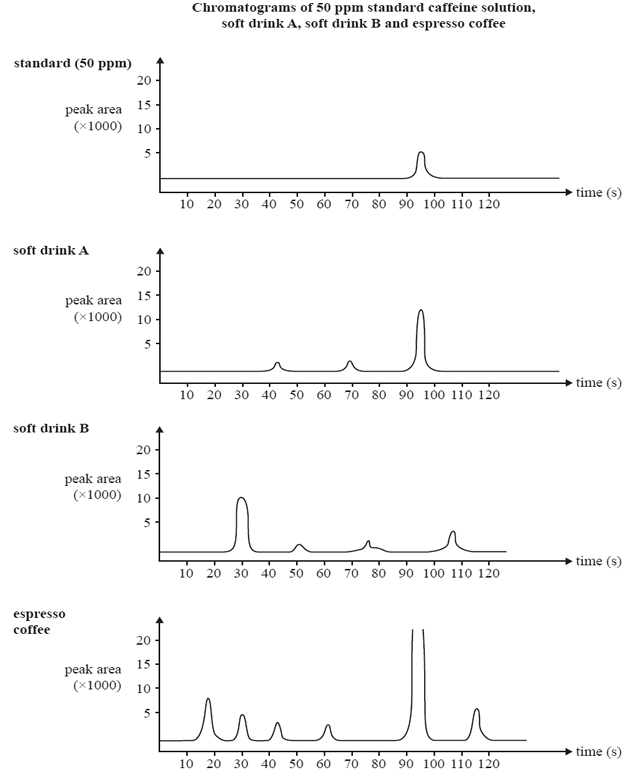
b) What evidence is presented in the chromatogram that supports the conclusion that soft drink B does not contain any caffeine?
Solution
c)
Explain why the caffeine content of the espresso coffee sample cannot be reliably determined using the information provided.
Solution
d) Describe what can be done to the espresso coffee sample so that its caffeine content can be reliably determined using the information provided.
Solution
