sample of uranium metal?
A. mass spectroscopy
B. gas liquid chromatography
C. atomic absorption spectroscopy
D. nuclear magnetic resonance spectroscopy
Solution
2) When a sample absorbs infrared radiation
A. covalent bonds are broken.
B. covalent bonds stretch and vibrate.
C. the spin alignment of certain nuclei changes.
D. electrons in atoms move to higher energy levels.
Soluti on

3) The graph shows the absorption spectra of three food dyes: Blue No. 1, Red No. 2 and Yellow No. 4.
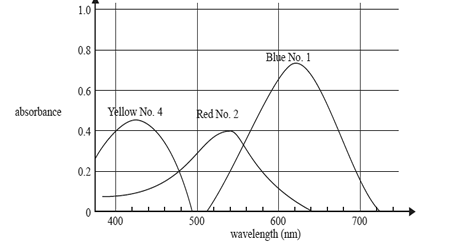
Which one of the following is the best wavelength to determine the concentration of Red No. 2 dye in a solution containing a mixture of all three dyes?
A. 430 nm
B. 500 nm
C. 540 nm
D. 620 nm
Solution

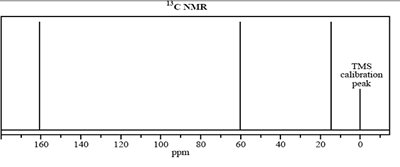
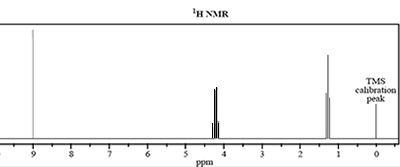
4) The molecular formula of an unknown compound, X, is C3H6O2.
The infrared 13C NMR and 1H NMR spectra of this compound are shown below.
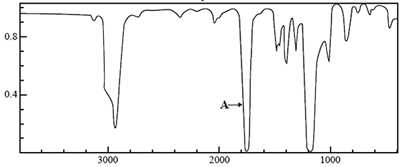
Using the Infrared absorption data on page 7 of the Data Book, identify the atoms and the bonds between them that are associated with the absorption labelled A on the infrared spectrum.
Solution
b. How many different carbon environments are present in compound X?
Solution
c. How many different hydrogen environments are present in compound X?
Solution
d. i. The signal at 1.3 ppm is split into a triplet. What is the number of equivalent protons bonded to the adjacent carbon atom?
Solution
ii. Draw the grouping of atoms that would give rise to the triplet and quartet splitting patterns.
Solution
e. A chemical test showed that compound X does not react with a base. Propose a structure for compound X that is consistent with all the evidence provided.
Solution
