1)Water is released during a polymerisation reaction.
Which monomer is likely to have been involved in the reaction?
(A) Ethene
(B) Glucose
(C) Styrene
(D) Vinyl chloride
Solution
2) How many isomers does this CH3CF3 compound have?
(A) 1
(B) 2
(C) 3
(D) 4
Solution
3) An organic liquid, when reacted with concentrated sulfuric acid, produces a compound that decolourises bromine water.
What is the formula of the organic liquid?
(A) C6H12
(B) C6H14
(C) C6H11OH
(D) C5H11COOH
Solution

4) The table shows information about three carbon compounds
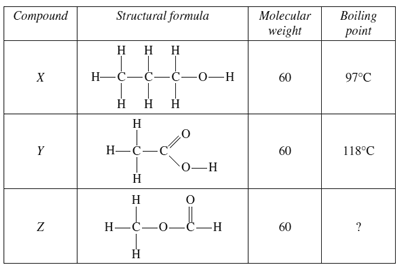
What is the best estimate for the boiling point of compound Z?
(A) 31°C
(B) 101°C
(C) 114°C
(D) 156°C
Solution
5) A student prepared the compound methyl propanoate in a school laboratory.
(a) Give a common use for the class of compounds to which methyl propanoate belongs.
Solution
(b) In the preparation of this compound a few drops of concentrated sulfuric acid were added to the starting materials. The mixture was then refluxed for a period of time.
Why was it necessary to reflux the mixture?
Solution
(c) Name the TWO reactants used in preparing the methyl propanoate and draw their structural formulae.
Solution

6) In the margarine industry, alkenes are often hydrogenated to convert unsaturated oils into solid fats that have a greater proportion of saturated molecules.
(a) Using ethene as an example, write an equation for this reaction and state the type of reaction this represents.
Solution
(b) Describe a test that could be used to confirm that all the ethene has been converted.
Solution
7) Compare the process of polymerisation of ethylene and glucose. Include relevant chemical equations in your answer.
8) Explain the relationship between the structures and properties of THREE different polymers from ethylene and glucose, and their uses.
