The following titration curve was obtained by measuring the pH in a reaction flask during an acid-base titration.
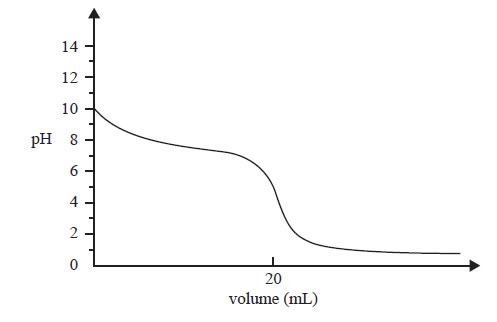
Question 1) The graph represents the change in pH in the reaction flask when
A. a weak acid is added to a strong base.
B. a weak base is added to a strong acid.
C. a strong acid is added to a weak base.
D. a strong base is added to a weak acid.
Solution
Question 2) Which one of the following is a suitable indicator for use in this titration? Click for the data sheet information.
A. phenol red
B. thymol blue
C. phenolphthalein
D. bromophenol blue
Solution

Question 3) A sample of the insecticide dichlorodiphenyltrichloroethane (DDT),
C14H9Cl5, was found to contain 0.120 g of carbon.
What mass of chlorine was present in the sample?
A. 0.127 g
B. 0.355 g
C. 0.994 g
D. 1.01 g
Solution
Question 4 When 1.0 mole of Cu3FeS3 and 1.0 mole of O2 are mixed and allowed to react according to the equation
2Cu3FeS3(s) + 7O2(g) → 6Cu(s) + 2FeO(s) + 6SO2(g)
A. no reagent is in excess.
B. 5 mole of O2 is in excess.
C. 5/7 mole of Cu3FeS3 is in excess.
D. 2/7 mole of Cu3FeS3 is in excess.
Solution
Click to see the assessors report
Question 5
One possible reaction that occurs when trinitrotoluene (TNT), C7H5N3O6, explodes is
2C7H5N3O6(s) → 2C(s) + 12CO(g) + 5H2(g) + 3N2(g)
When one mole of TNT explodes the total volume of the gases produced from this reaction, measured at 27 °C and 1.00 × 102 kPa, is closest to
A. 0.249 L
B. 22.7 L
C. 249 L
D. 274 L
Solution
Click to see the assessors report
