Consider the following TLC plate of compounds X, Y and Z developed using a suitable mobile phase on a polar stationary phase.
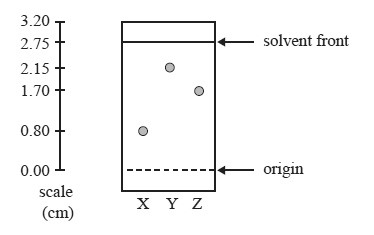
Question 1) The Rf value of the most polar component in this TLC separation is
A. 0.29
B. 0.62
C. 0.78
D. 0.80
Solution
Question 2) A forensic chemist wants to test the accuracy of a gas chromatograph that is to be used for the analysis of blood alcohol
content.
A blood sample may contain a number of volatile chemicals that can interfere with the identification and measurement of ethanol in the blood. A sample containing a mixture of ethanol and several other volatile chemicals was injected into the gas chromatograph. The following chromatogram was obtained.
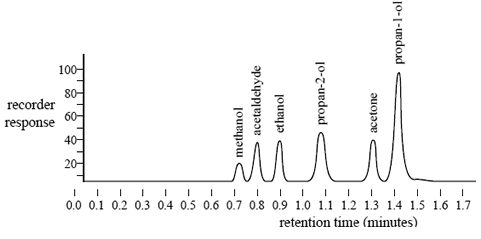 The forensic chemist claims that the presence of these volatile chemicals would not affect the qualitative analysis of ethanol.
The forensic chemist claims that the presence of these volatile chemicals would not affect the qualitative analysis of ethanol.
i. What evidence is presented in the chromatogram to support this claim?
Solution
ii. To determine the percentage of alcohol in a blood sample only the peak at a retention time of 0.9 minutes is measured. Explain why.
Solution
Click to see assessor's report

Question 3) The following calibration graph was constructed using simulated standard blood alcohol samples ranging in concentration
from 0.000% to 0.400% m/v ethanol. Each standard was run through the chromatography column and the area under the peak produced at a retention time of 0.9 minutes was measured.
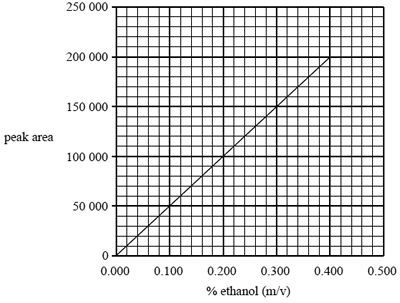
The blood alcohol content of a car driver was determined using this chromatographic technique.
Determine the percentage (m/v) of alcohol in the driver's blood if the peak area at a retention time of 0.9 minutes was found to be 110 000.
Solution
