The following table lists the pH of 0.10 M solutions of four different acids at 25°C.
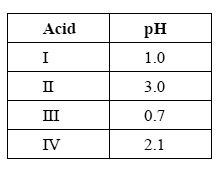
Which one of the four acids listed in the table above has the smallest Ka value?
Solution
Which acid must have more than one acidic hydrogen per molecule? Give a reason for your answer.
Solution
Using the concentration and the pH of acid IV, calculate the percentage ionisation of acid IV in the 0.10 M solution.
Solution
Calculate the value of the ratio [OH–] acid II / [OH–] acid I present in the solutions of acids II and I.
Solution
Samples of the solutions of acids I and IV are diluted by a factor of 10.
The resulting change in pH units would be
Give an explanation for your answer.
Solution

Data sheet is provided for these questions.
Methanoic acid is a weak monoprotic acid.
Calculate the concentration of a methanoic acid solution that will have the same pH as acid IV.
Solution
The dissociation of methanoic acid in water is exothermic. If a solution of the acid is heated, will the pH of the solution increase, decrease or remain constant?
Give an explanation for your answer.
Solution
