Thermochemistry (2005 VCE)
The graph below represents the energy changes over the course of a chemical reaction
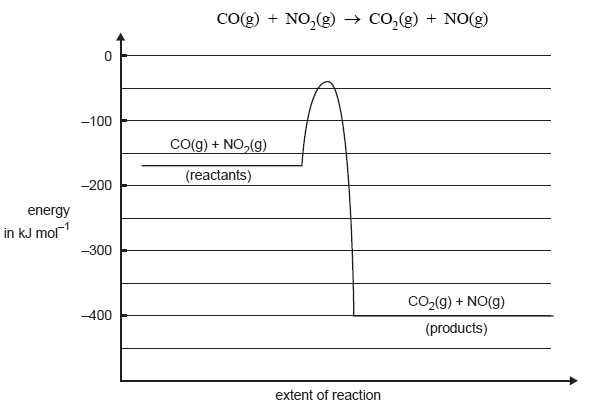
Give the magnitude and sign of the ΔH for the forward reaction in kJ/mol.
Solution
Give the activation energy for the reverse reaction in kJ mol1.
Solution
Give two reasons explaining why the rate of this reaction increases with increasing temperature.
Solution

A suitable catalyst is discovered for the reaction. What would be the likely effect of the catalyst on:
- the activation energy? Explain your answer.
Solution
- the ΔH? Explain your answer.
Solution
