Sulfuric acid can be produced from mined sulfur via the Contact Process. The first two stages in the industrial production of sulfuric acid by this process are represented below.
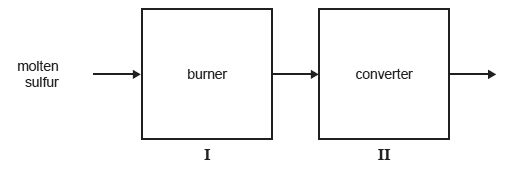
Give a reason why, in stage I, the molten sulfur is sprayed into the burner rather than being allowed to flow through it.
Solution
A conflict is involved in choosing the best temperature to be used in stage II, where the reaction is 2SO2(g) + O2(g) => 2SO3(g)
Describe the nature of the conflict and explain how the conflict is resolved.
Solution
Would increasing the pressure of the reacting mixture in the converter affect the amount of SO3 produced in stage II? Explain your answer.
Solution

Sulfuric acid is a diprotic acid. The first ionisation reaction of sulfuric acid is complete while its second ionisation is that of a weak acid. Give chemical equations for both the first and second ionisation reactions
of sulfuric acid.
Solution
Give one major industrial use of sulfuric acid
Solution
