Particle theory
Diffusion
Diffusion is the spreading of a substance when placed in another . Diffusion is most rapid in gases due to the high speed of the particles and the immense distance between them. It is somewhat slower in liquids and even slower, if at all, in solids. That is because the particles of a solid have very little kinetic energy, in other words the particles have very little motion.
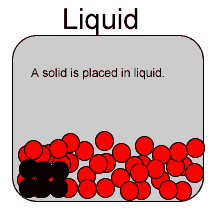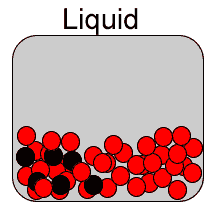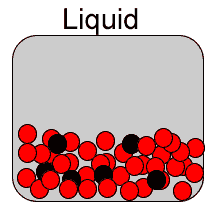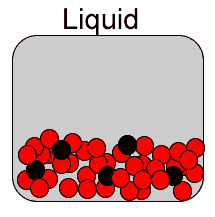
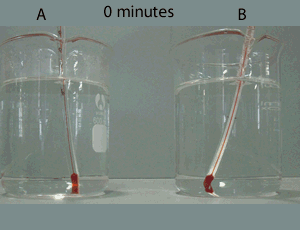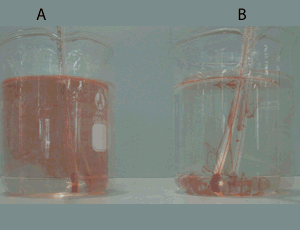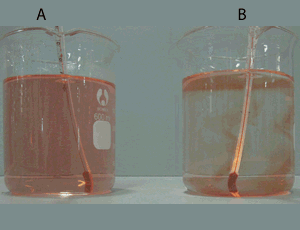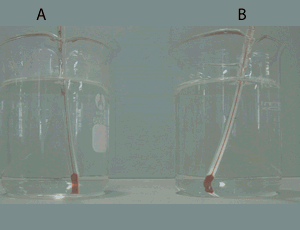
Now consider the video on the right. It shows how red food dye diffuses in warm (A) and cold (B) water. The process is a lot slower than if both water and the dye were gases as explained above.
Why is the rate of diffusion of the dye particles slower than if both the water and dye particles were in the gas state?
Consider the video on the right. The red coloured water is not mixing with the blue water. It will stay like this for a up to 10 minutes. Knowing that one of these two samples of water is hotter than the other and that less dense substances float on the surface of liquids that are more dense:
- identify which is the hotter sample of water. Give a reason for your selection.
-
explain using particle theory why the two samples of water do not mix.
Sourced from https://www.youtube.com/watch?v=H0xB15fNzHc 7.29pm 12/07/20
Continue with diffusion demonstration with Skittles.
Continue with density