Numerous enzymes rely on cofactors to exhibit catalytic activity. These cofactors can manifest as either coenzymes or inorganic ions, like Ca2+ ions. Coenzymes are small organic molecules, often derived from vitamins, that certain enzymes necessitate for their functionality. Vitamins constitute organic compounds essential in minute quantities within animal diets. Coenzymes accept electrons or small molecular groups and, in contrast to enzymes, undergo modifications during the reaction. In subsequent reactions, coenzymes can be reset to their initial states by eliminating the electrons or small molecules they have acquired.
Vitamins are imperative in minuscule measures and, apart from vitamin D—synthesized by the body—must be ingested through animal diets, making them indispensable nutrients. Two vitamin categories exist: water-soluble and fat-soluble. Water-soluble vitamins circulate freely throughout the body and are eliminated via the kidneys. Since they are continually flushed out, water-soluble vitamins are required daily and are unlikely to attain toxic levels compared to fat-soluble vitamins.
Conversely, fat-soluble vitamins are stored within the body and are not as easily excreted as their water-soluble counterparts. Daily intake isn't obligatory for fat-soluble vitamins, unlike water-soluble ones. An excess of fat-soluble vitamins can lead to toxicity, as they accumulate within animal bodies, notably in the liver.
Fat soluble vitamins can be distinguished from their chemical structure. Consider vitamin D, shown on the right. It is predominantly a hydrocarbon with one lone OH group at the end. This makes the molecule hydrophobic as it is unable to interact with the polar water molecules and allows for a high degree of interaction between lipid molecules. |
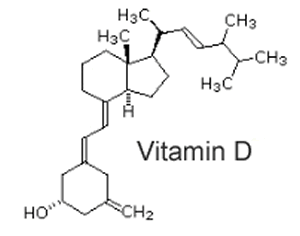 |
| Consider the molecule of vitamin C, shown on the right. In contrast to the lipophilic (fat loving) vitamin D molecule, this has a number of polar (OH) groups protruding from the molecule. Hydroxy groups create hydrogen bonding between the vitamin C molecule and water molecules. This high degree of interaction with the water molecules allows the molecule to be highly soluble in water. |
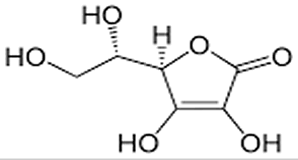 |
1) Consider the molecule of vitamin A, shown on the right. a) Is this vitamin hydrophobic or hydrophilic? b) Is it likely to be required in the diet on a daily basis? c) Is this molecule rapidly excreted via the kidneys? d) Is it toxic in large doses? Solution |
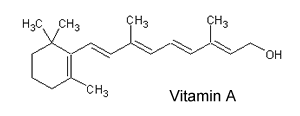 |
| 2) Vitamins are sometimes called coenzymes. Why is this strictly incorrect? Solution |
 |
3) Consider the three molecules shown on the right. a) Identify the three molecules shown on the right as hydrophobic or hydrophilic? b) Which are likely to be required by the body on a daily basis? c) Which woulld be expected to appear in the urine soon after ingestion?
|
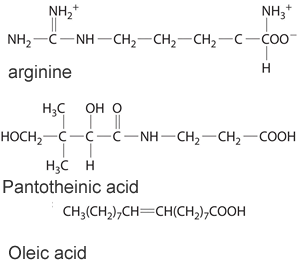 |