Hide solution
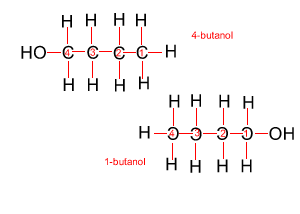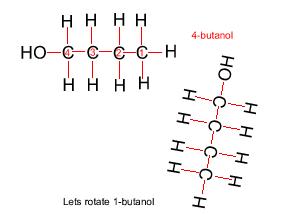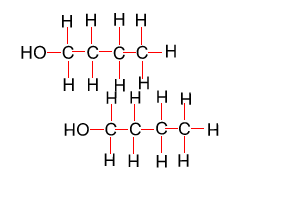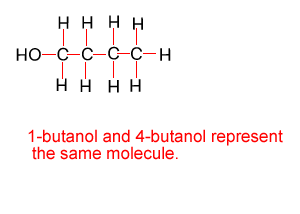
Because atoms can rotate about single covalent bonds 1-butanol is the same molecule as 4-butanol and 2-butanol is the same molecule as 3-butanol.
Hide solution
Because atoms can rotate about single covalent bonds 1-butanol is the same molecule as 4-butanol and 2-butanol is the same molecule as 3-butanol.

| Chemistry of cellulose and ironing |
|
|
Fabrics made of cellulose fibers exhibit good structural strength and aintain their shape when dry, however, they shrink or wrinkle when wet. |
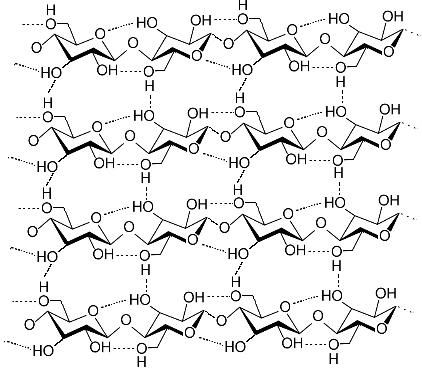 |
If the fabric is wet, however, wrinkling and shrinkage can occur. This occurs becasue water molecules disrupt the hydrogen bonds between the cellulose chains When the hydrogen bonds are broken cellulose the cellulsoe chains move relative to each other. As tghe fabric dries out hydrogen bonds between the cellulose chains reform and once again hold the chains tigthtly in their new position. Permanent Press shirts are shirts that never need ironing. They have permanent creases and folds thanks to permanent crosslinks that exist between the cellulsoe chains. These crosslinks hold the chains in permanent position and prevents movement which causes wrinkles and shrinkage.
|
 |
Ironing uses heat and steam to break hydrogen bonds and reposition the displaced cellulose chains back to their original position. |