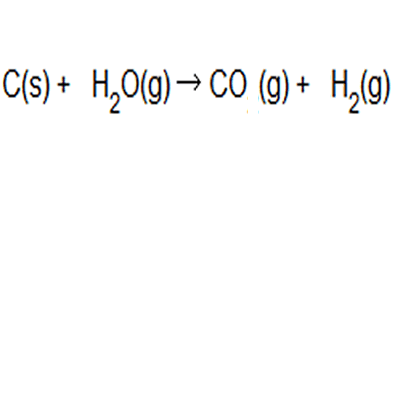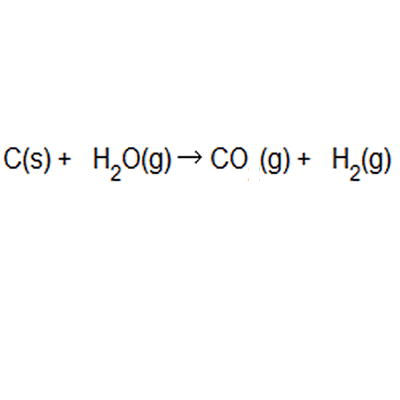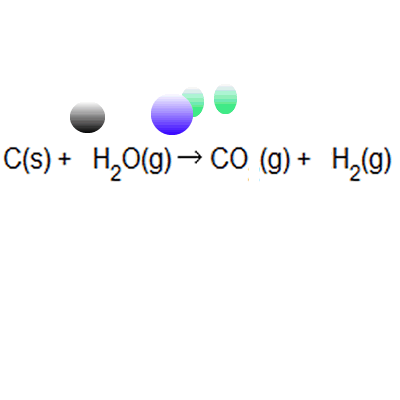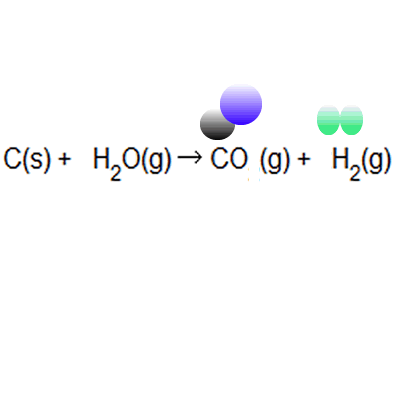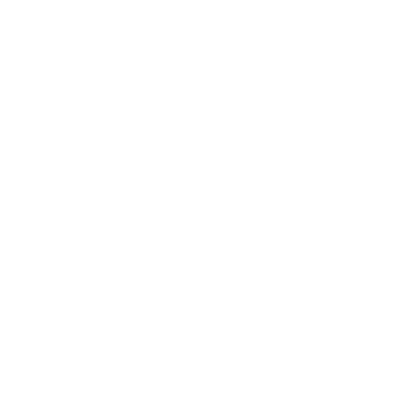
Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy is the second principle of green chemistry
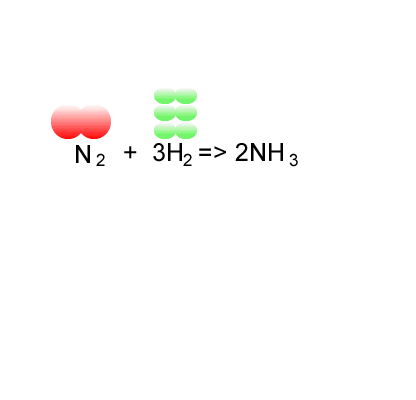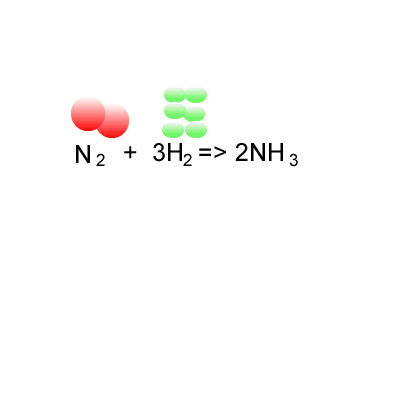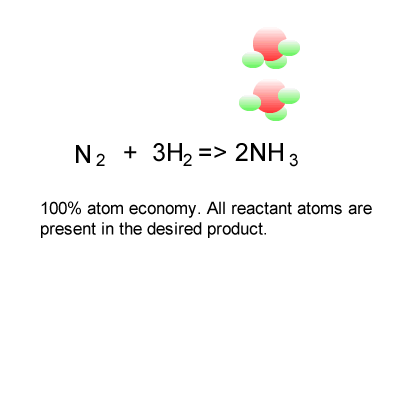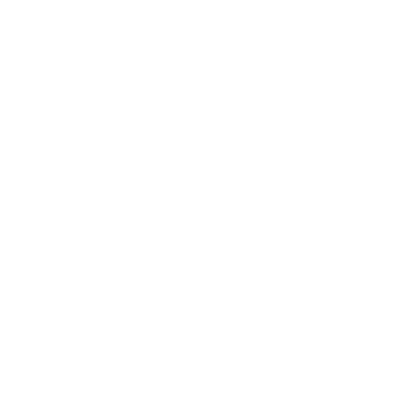
The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product . In other words, atom economy is a calculation which measures "how much of the reactant atoms actually form the final product", the higher the atom economy the lower the amount of waste product formed.
Percentage atom economy is really a measure of the sustainability of a chemical reaction. In other words, wasteful a reaction is. It gives and indication of the amount of waste products produced during a reaction in forming a desired product.
Percentage yield tells us the amount of the reactants that should react to form the product have successfully converted into products. Atom economy on the other hance, tells us how many atoms in the total number pf reactant atoms will be converted into our desired product.
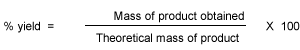
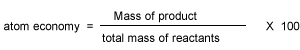
Consider the combustion of methane to form carbon dioxide and water.
CH4(g) + 2O2(g) => 2H2O(g) + CO2(g)
In this particular reaction 32.0 grams of methane burns completely to produce 66.0 grams of water. What is the percentage yield?
Step 1 Convert mass of methane into mol of methane
=> 32.0 /16.0 = 2.00 mol
Step 2 Calculate the mol of water 2.00 mol of methane will produce according to the stoichiometry
=>
2 X 2.00 = 4.00 mol.
Step 3 Calculate the theoretical mass of water produced
=> 4.00 X 18.0 = 72.0 grams
Step 4 Calculate the % yield
=> (66.0/72.0) X 100 =
91.7%
What is the atom economy of the reaction below in producing water?
CH4(g) + 2O2(g) => 2H2O(g) + CO2(g)
Step 1 Calculate the total mass of reactants as per equation.
=> 16.0 + 2 X 32.0
= 80.0 g
Step 2 Calculate the mass of water as per equation
=> 2 X 18.0 = 36.0 g
Step 3 Calculate the atom economy
=>
(36.0 / 80.0 ) X 100 = 45.0%
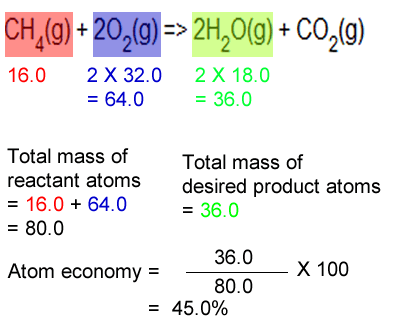
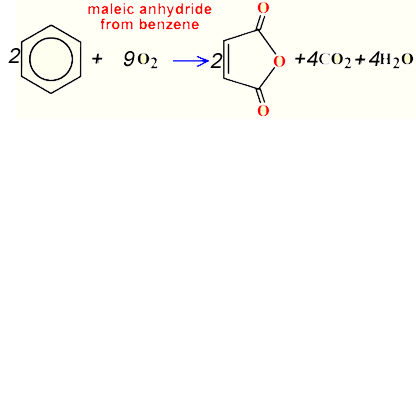
Consider the synthesis of maleic anhydride from benzene as shown on the right.
Calculate the atom economy for this reaction.
Solution
Hydrogen is made by two complete processes. One involves reaction of steam and coal and the other involves reaction between methane and steam
C(s) + 2H2O(g) → CO2(g) + 2H2(g)
CH4(g) + H2O (g)→ CO(g) + 3H2(g)
What is the atom economy for making hydrogen by reacting coal with steam according to the equation above?
Solution
What is the atom economy for making hydrogen by reacting methane with steam according to the equation above?
Solution
A low atom economy indicates that the reaction is
Methane gas can be produced through microbial breakdown of organic waste. Which reaction should be used to produce hydrogen gas?
Give two reasons.
Solution

Consider the formation of ammonia via the Haber process.
Nitrogen is reacted with hydrogen to produce ammonia, according to the equation below.
N2(g) + 3H2(g) => 2NH3(g)
The hydrogen, however, comes from the reaction between methane and water according to the equation below.
CH4(g) + H2O (g)→ CO(g) + 3H2(g)
Calculate the atom economy for the formation of ammonia.
Solution

Hydrogen gas is produced from coal and steam in a controlled process to produce carbon monoxide and hydrogen gas. The equation to this chemical reaction is shown on the right. What is the atom of economy with respect to hydrogen gas production?
Solution