Molecular shape
of
carbon dioxide (CO2)
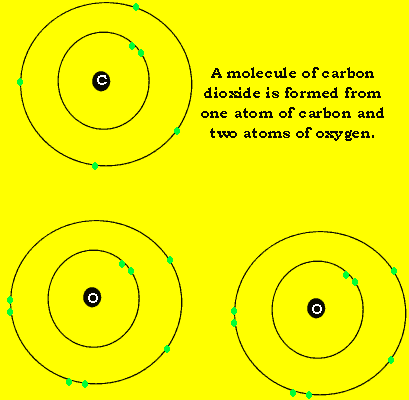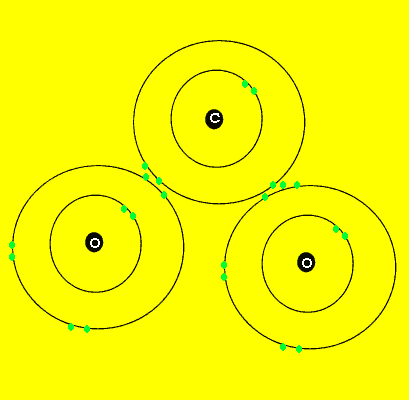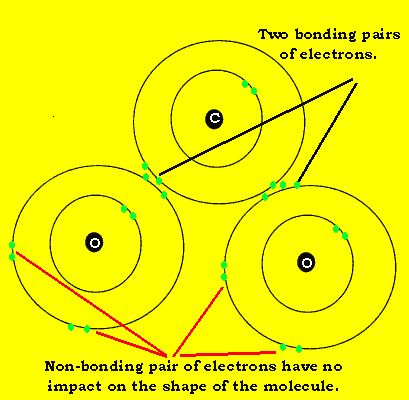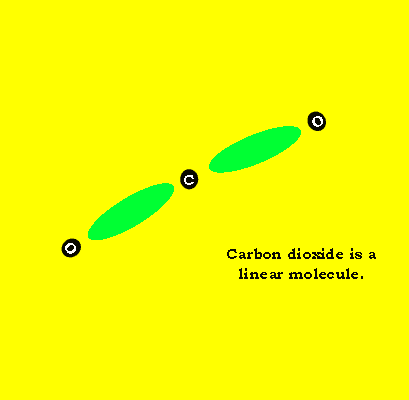
Carbon dioxide, like water, is composed of three atoms. Unlike water, carbon dioxide is a linear molecule. Look at the animation on the left.
Each double bond is considered as one charge cloud. So we have two charge clouds to repel each other. The maximum distance theycan move apart is move to opposite sides of the central carbon atom. This creates a linear molecule.
Continue.
with PCl5 and SF6