Electrons in an atom prefer to exist in pairs. The pairs of electrons in the atom occupy certain volumes of space around the nucleus called charge clouds. Having a negative charge these clouds repel each other in 3D space around the central atom, as shown on the right. This is called the valence shell electron pair repulsion (VSEPR) and dictates the shape of the molecule.
Lets see with some examples.
Lets take ammonia (NH3) first.

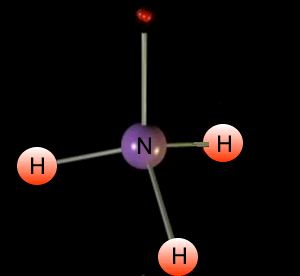
Look at the animation on the left. It shows the formation of ammonia. Ammonia is a molecule composed of one nitrogen and three hydrogen atoms.
The central atom is nitrogen
and it is the number of electron pairs, both bonding and non-bonding,
positioned around nitrogen that determines the shape of the molecule.
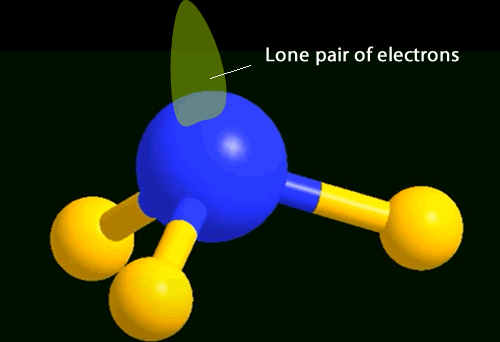
You will notice 4 pairs of
electrons around the central nitrogen atom. Three bonding and one non-bonding,These
pairs of electrons form regions of negative charge (electron clouds) that
repel each other. They move as far apart as possible forming a tetrahedral
shape.
Click to see a video of the ammonia molecule.
The shape of the molecule is defined by the position of the atoms present around the central atom.
In the case of ammonia the shape is a trigonal pyramid.