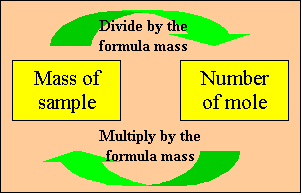
The number 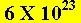 is
calculated so that a mole of any compound weighs as much as its molecular
formula expressed in grams.
is
calculated so that a mole of any compound weighs as much as its molecular
formula expressed in grams.
For example, the formula mass of ethane(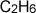 )
is 18 atomic mass units. The mass of
)
is 18 atomic mass units. The mass of 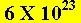 ethane
molecules weigh 18grams.
ethane
molecules weigh 18grams.
The atomic masses of some elements are listed
C=12, O=16, N=14, Be=4, H=1.