
Heat is used in cooking to speed up the many chemical reactions that result in a delicious meal. All matter consists of atoms joined together to form different substances. The point at which these atoms join together is known as a chemical bond. During a chemical reaction chemical bonds are broken and then reformed to make new products. We often use heat to break chemical bonds and get the reaction going.
The animation on the right shows how heat is used to speed up the particles and break them apart. The atoms then rejoin to form a new product.
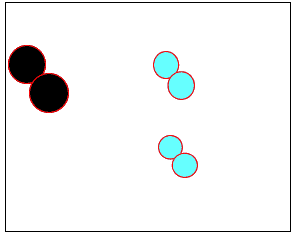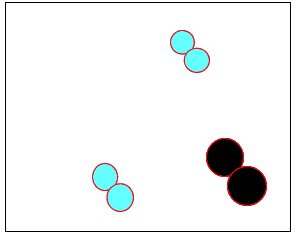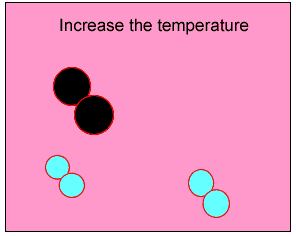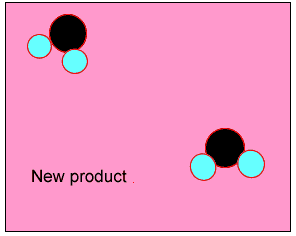
Normally the particles above travel too slowly to react together. With the increase in heat the particles travel faster, colliding with greater force. Chemical bonds are broken and then reformed between different atoms, creating new substances.

This video shows a chemical reaction producing carbon dioxide gas when cold water is used. Exactly the same amount of powder and water was used as in the hot water test shown in the video above.
What do you notice about the speed of the reactions?