S.T.P.
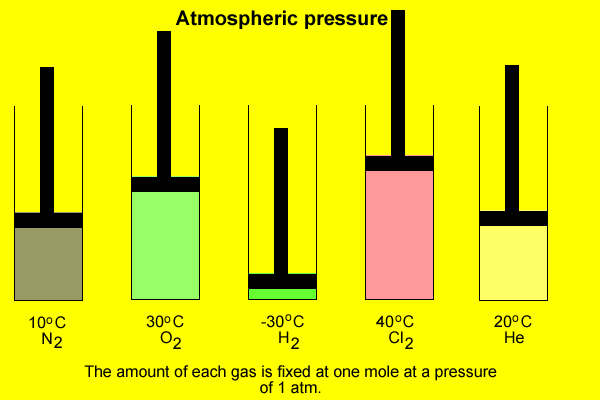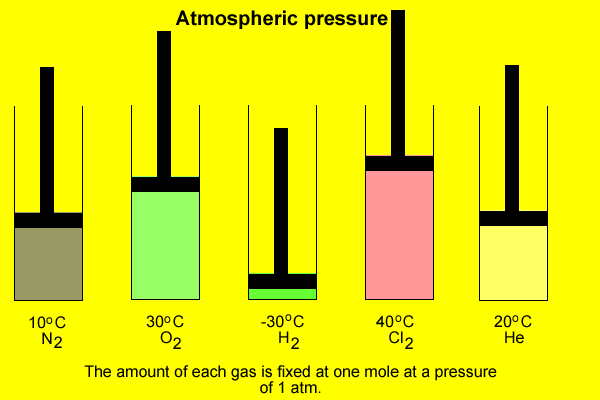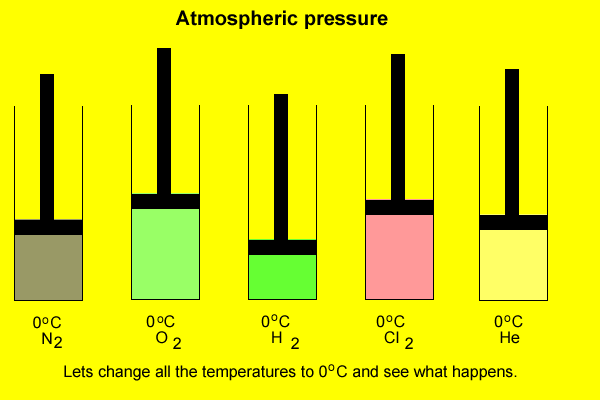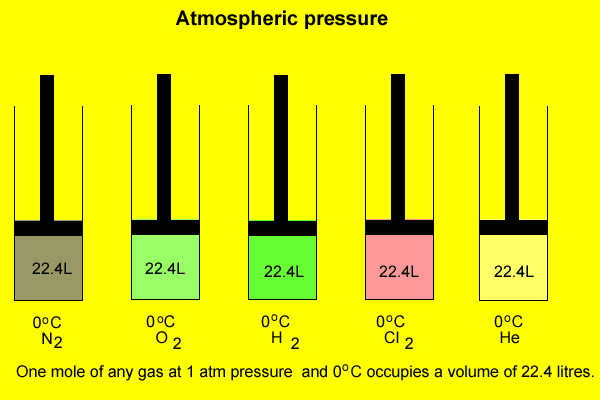
The volume occupied
by one mole of any gas
at standard temperature(0o) and pressure(1 atm) is 22.4 litres.
This volume is a close approximation and different gases vary slightly.
The molar volume of any gas at S.T.P. is 22.4L .The mole of any gas can therefore be found using the expression on the right. Lets do some examples.
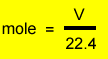
Example 1
Calculate the number
of mole of hydrogen gas that occupies a volume of 2.24L at S.T.P.
=> mole = 2.24/22.4 = 0.1
Example 2
Calculate the mass of oxygen gas(O2) that expands to a volume
of 33.6L at 0oC and 1 atm pressure. Atomic mass O =16.
The conditions are obviously at S.T.P. so we can use the formula above.
Step 1) Find the number of mole of oxygen gas.
=> mole = 33.6/22.4 = 1.5
Step 2) Find the mass of oxygen gas represented by 1.5 mole.
=> mass = mole X molar mass = 1.5 X 32 = 48 grams
Example 3
12 grams of hydrogen gas(H2) is placed in a piston at 0oC
and 760mmHg pressure. What is the volume to which the piston expands?
Atomic mass H=1
The conditions are S.T.P. temperature is 0oC and pressure is
760mmHg = 1atm.
Step 1) Convert the expression above to make it equal to V
=> V = n X 22.4L
Step 2) Calculate the mole of hydrogen
=> mole = mass / molar mass = 12 /2 = 6 mole
Step 3) Calculate the volume
V = 6 x 22.4L = 13.4L
Try these exercises.
1) What is the volume occupied by 3.5 mole of nitrogren gas as S.T.P?
Answer
2) What mass of nitrogen gas(N2) occupies a volume of 11.2L
at 0oC and 760mmHg pressure. (Atomic mass N=14)
Answer
3) How many mole of helium gas should be placed in a piston so that it
expands to fill a volume of 56.8L at S.T.P.?
Answer
4) 35 grams of an unknown gas
occupies a volume of 11.2 litres at S.T.P. Scientists suspect nitrogen(N2),
oxygen(O2), chlorine(Cl2) or hydrogen(H2)
gas. Which one is it likely to be?
Atomic mass H=1,O=16,Cl=35.5,N=14.
Answer