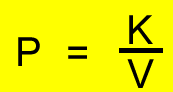Click
to hide the solution
P1V1 = P2V2
P1 = 250kPa
V1 = 20
L
P2 = ?
V2 = 100L
=> 250 X 20 = P2 X 100
=> 5000/100 = P2
=> 50 kPa = P2
Click
to hide the solution
P1V1 = P2V2
P1 = 400kPa
V1 = 5
L
P2 = 80
V2 = ?
=> 400 X 5= V2 X 80
=> 2000/80 = V2
=> 25 = V2 this is the combined volume of the two vessels
So the volume of the larger vessel is 25 -5 = 20 litres
Boyle's
Law
Exercises
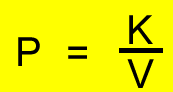
|
|
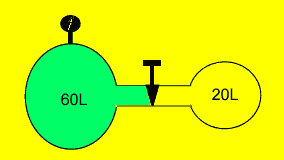
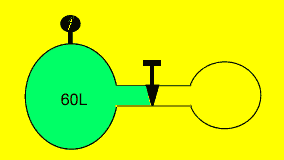
A fixed amount of gas was sealed
in a 60 litre vessel at 60 kPa. The valve separating the 60 litre vessel
from a 20 litre vessel, as shown on the right, was opened and the gas
allowed to flow freely. Calculate the pressure exerted by the gas on the
vessel wall assuming the temperature is constant.
Solution
|
|
|
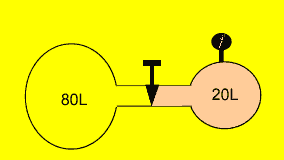
An unknown gas is kept in a
20 litre vessel at 250kPa. The valve is opened and the gas moves freely
into the adjoining 80 litre vessel. What is the final pressure of the
gas if the temperature remains constant?
Solution
|
|
|
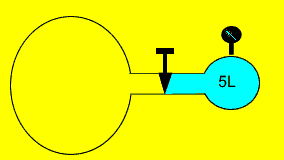
Oxygen gas is kept in a 5 litre
vessel at 400kPa. The valve is opened and the gas is allowed to flow freely
between the two chambers, as shown on the right. The pressure drops to
80 kPa and the temperature remains constant. Calculate the volume of the
adjoining vessel.
Solution
|
|
|
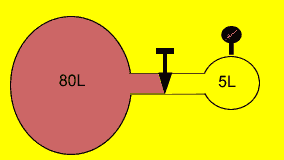
An 80 litre
flask of oxygen gas is connected by a stopcock to an empty 5 litre flask,
as shown on the right. When the stopcock is opened the gas expands into
the combined volume of the two containers. The pressure gauge attached
to the 5 litre flask now reads 80kPa. Assuming constant temperature what
was the pressure of oxygen in the 80 litre flask prior to opening the
stopcock?
Pass the mouse over the image to show the answer.
|
|
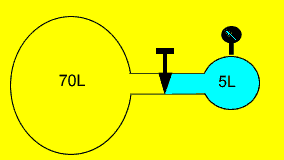
Helium gas is kept
in a 5 litre vessel at 350 kPa as shown on the right. The stopcock joining
the two vessels is opened and the gas expands to fill the combined volume
of both vessels. What is the reading on the pressure gauge after the stopcock
is opened? Assume constant temperature.
Pass the mouse over the image to show the answer. |
|
A 60 litre vessel
joined to a smaller, empty vessel by a valve contains nitrogen gas at 230
kPa. When the valve joining the two vessels is opened the pressure drops
to 180 kPa. What is the volume of the empty vessel if temperature remains
constant?
Pass the mouse over the image to show the answer. |
|
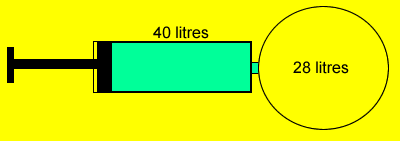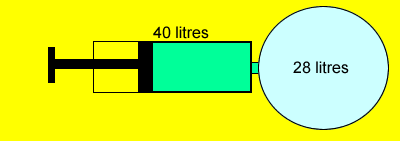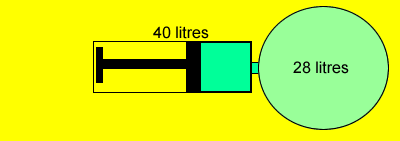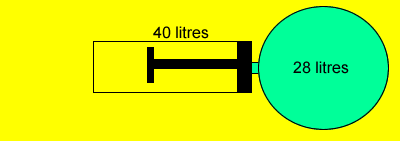 |
40 litres of helium
gas at 80 kPa is pumped into a 28 litre empty vessel. If the gas remains
at the same temperature what is the final pressure of the gas in the 28
litre vessel?
Pass the mouse over the image to show the answer. |