secondary cells and fuel cells.
| Fuels cells | Primary | Secondary | |
| Energy transformation when discharging | chemical --> mostly electrical + heat | chemical --> mostly electrical + heat | chemical --> mostly electrical + heat |
| Energy transformation when recharging | Recharging is not possible | Recharging is not possible | electrical --> mostly chemical + heat |
| Anode during discharge | Site of oxidation (negative) | Site of oxidation(negative) | site of oxidation(negative) |
| Anode during recharge | Recharging is not possible | Recharging is not possible | Site of oxidation(positive) |
| Chemical reactions when discharging | Spontaneous and exothermic | Spontaneous and exothermic | Spontaneous and exothermic |
| Chemical reactions when recharging | Recharging is not possible | Recharging is not possible | Reactions are endothermic as energy is supplied to drive the reactions taking place at the electrodes. |
| Reactants | Constantly supplied | Finite amount present in the battery | Finite amount present in the batter |
| Products | Constantly removed | Stored in the battery away from electrodes | Stored in the battery but in contact with the electrodes |
| Electron flow during discharge (always anode to cathode for all electrochemical cells) | Anode to cathode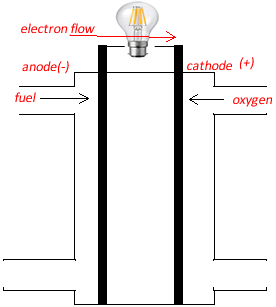 |
Battery discharging electron flow anode to cathode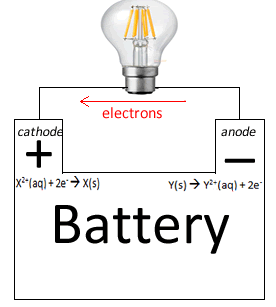 |
Battery discharging anode to cathode
|
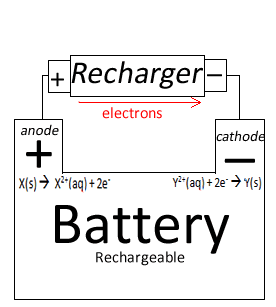
| Electron flow during recharge | Recharging is not possible | Recharging is not possible | Battery recharging anode to cathode
Notice when:
|
| Ion flow during discharge | Anions flow to the anode | Anions flow to the anode | Anions flow to the anode |
| Ion flow during recharge | Recharging is not possible | Recharging is not possible | Anions flow to the anode |
| Voltage used to recharge | Recharging is not possible | Recharging is not possible | Greater than the voltage output during discharge. |
| Voltage output | Consistent voltage as reactants are constantly supplied | Drops off as reactants are used up | Drops off as reactants are used up |