Any substance that is used as a fuel must produce a great deal of heat and gas.
Rocket fuels burn to produce a great deal of heat and gas. The heat causes the gas to expand. As the gas expands it is forced out of the engine nozzle and forces the rocket upwards.
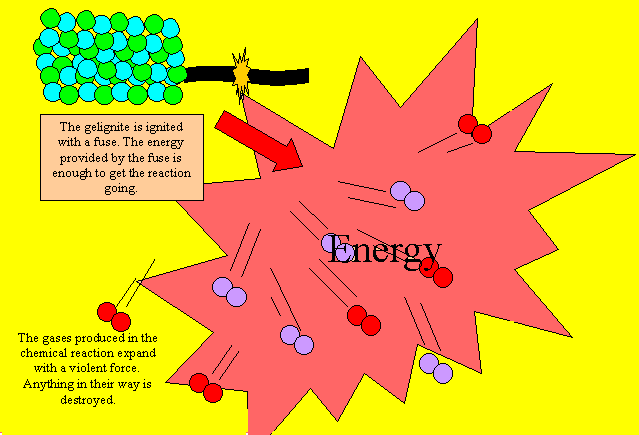
Gelignite works in much the
same way as rocket fuels only quicker, much, much quicker. When gelignite
is ignited the reaction produces gases and heat very quickly. As the gas
violently expands it pushes aside anything in its way with incredible
force. This reaction is too violent and quick to be used as a fuel for
rockets.

The Chinese were the first to use rockets, primarily as fireworks displays
and as weapons. Such rockets consisted of bamboo filled with a mixture
of sulfur, charcoal, and potassium nitrate commonly known as saltpeter.
This mixture is more commonly known as gunpowder.
Almost all rocket propellants consist of an oxidant and a fuel. An oxidant is a substance that, generally, decomposes to produce oxygen when heated. In the case of gunpowder, the fuel is charcoal and the oxidizer is the potassium nitrate. Sulfur is used to speed up the reaction and acts like a catalyst in that it increases the rate of reaction. However, unlike a catalyst, it is used up in the reaction.
Modern day solid fuels consist of ammonium perchlorate powder (an oxidizer), mixed with fine aluminium powder (a fuel). The contents are held together with a rubbery material which burns during launch. It is hard to imagine that metals, such as aluminium, are used as fuels, but metal powder is very flammable when mixed with an oxidant. Click to see how a mixture of metal powders and an oxidant can burn with a violent release of heat.

One
of the best fuels ever tested in a rocket was a mixture of liquid lithium,
liquid hydrogen and liquid fluorine. However the use of such fuels is
very impractical. For example to keep all three components in liquid form
we need to keep:
- hydrogen below -250 oC
- lithium above 180 oC
- lithium in an air tight container as it reacts explosively with air
and is highly corrosive.
The exhaust from this mixture is highly toxic.
What is an oxidant?
An oxidant does not always decompose upon heating to produce oxygen, as mentioned above. Sulfur reacts with zinc in a very fierce exothermic (heat producing) reation. You can view this reaction by clicking the link below. Sulfur is said to be a powerful oxidant. A more general description of an oxidant is that of a substance the readily takes electrons from another chemical. A chemical that gives electrons to the oxidant is called a reductant.
The
Space Shuttle burns oxygen with hydrogen to produce water according to
the equation
2H2(g) + O2(g) => 2H2O(g).
Oxygen takes electrons from the hydrogen, so oxygen is the oxidant and
hydrogen is the reductant. We must keep the oxidant and reductant separated
from each other until we are ready for launch where upon each chemical
is pumped into the ignition chamber and spontaneously react.
Continue with an example of an exothermic reaction using sugar as a fuel.
Continue with an example of an exothermic reaction using zinc and sulfur as a fuel.
Continue with an example of an exothermic reaction using hydrogen peroxide as a fuel.
Continue with an example of an exothermic reaction using metal powders as a fuel.
The reactions all proceed
Each reaction produces
These reactions are categorised as
Chemical reactions that are used to launch rockets generally produce
Solid fuel engines
Zinc and sulfur react according to the equation 8Zn(s) + S8(s)
=> 8ZnS(s). This reaction is used to launch small model
rockets.
a) The amount of thrust produced would be significantly higher if
b) Zinc is the
c) Oxygen is the