Condensation
polymers
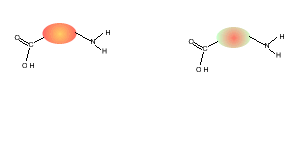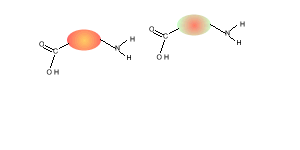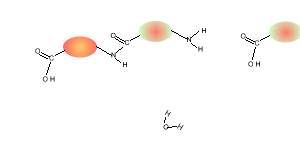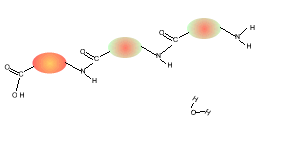
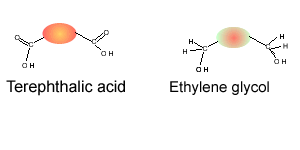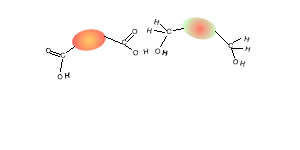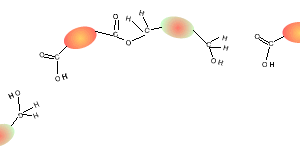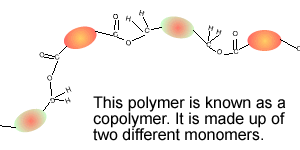
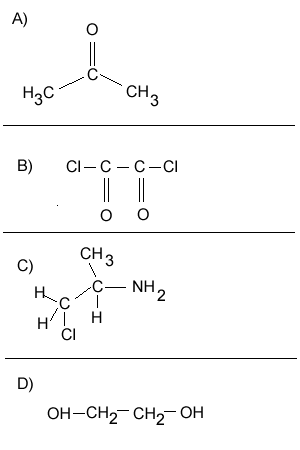
Consider the compounds shown on the right.
1) Which compound can act as a monomer, when used on its own, to form a condensation polymer?
2) What small molecule will be given off as each monomer links to the chain.
3) Which two compounds can act as monomers to form a condensation polymer?
4) What small molecule is given off when these two compounds react?
5) Draw the structural formula of the polymer formed in Q4. Solution