Fireworks, colour and the electron

The many colours visible during a fireworks display are due to electrons moving up and down the energy levels of the atom.

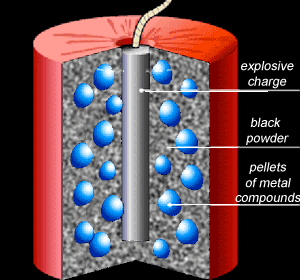
A typical fireworks shell contains an explosive mixture with pellets of metal compounds evenly distributed within it.
The explosive material blows the pellets in all directions while the black powder ignites the pellets and releases heat energy that is used to excite the electrons of the metal atoms to higher energy levels. Click on the link at the bottom to see a more detailed explanation of how colour is created. Colour is created according the metal compound present in the pellet. Copper compounds, for example, create brilliant green and blue colours.
This can be demonstrated in class by a simple flame test.