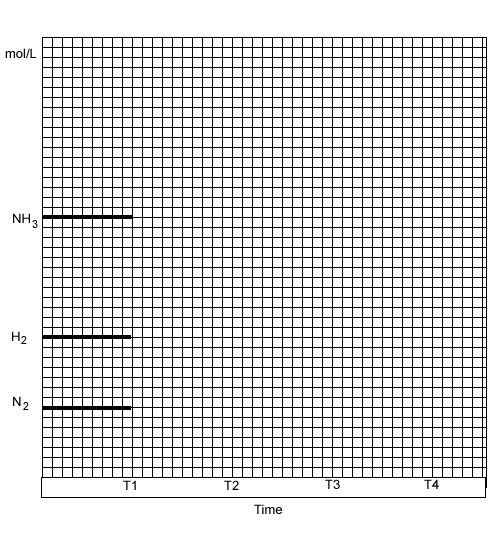
3H2(g) + N2(g) ![]() 2NH3(g) /\H
= -91 kJ/mol
2NH3(g) /\H
= -91 kJ/mol
| Action on the equilibrium | Equilibrium
constant Kc Click one of the options below |
Mol
of H2 when equilibrium is established |
Mol
of N2 when equilibrium is established |
Mol
of NH3 when equilibrium is established |
At
time T1 ddition of |
|
|||
| At time T2 the reaction chamber is doubled in volume | ||||
| At time T3 the reaction vessel is heated | ||||
| At time T4 t he reaction vessel is cooled | ||||
| At time T5 Addition of H2 |
On the set of axis provided below, sketch the concentration changes that occur when the changes above take place.
 |