Concentration and rates
Solution
Using the Haber process shown below indicate how the concentration and the rate will change as the systems shown below is stressed.
![]()
Draw how the system will respond by completing the graphs shown on the right when at :
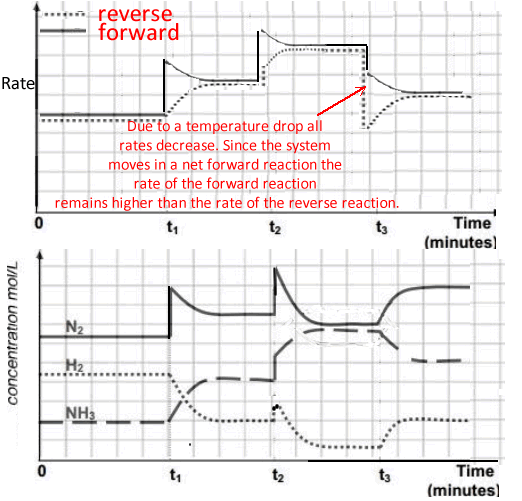
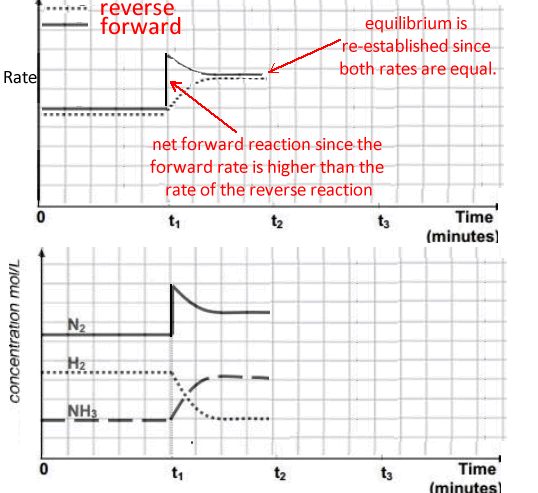
t1 - the concentration of nitrogen gas is increased to 9.0 M
The concentration of nitrogen should spike to 9.0M and the system should progress in a net forward reaction to partially undo the increase in the nitrogen concentration.
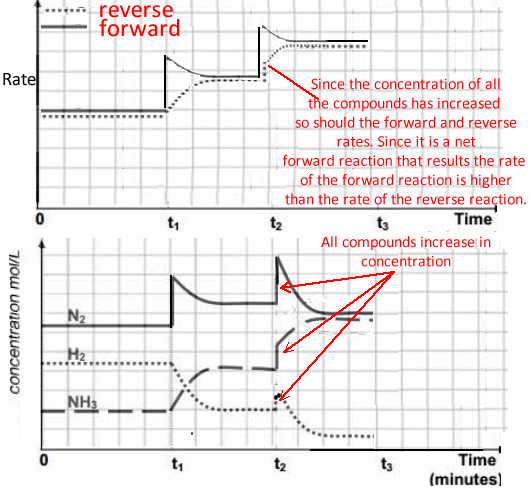
t2 - the volume of the reaction vessel is decreased
An increase in the concentration of all species in the system causes the reaction to move to the side of least particles. In this case it is a net forward reaction.
Both the forward and reverse rates increase, however, the rate of the forward reaction remains higher than the rate of the reverse reaction, thus giving a net forward movement to the reaction.
** Note. Changes to the concentration should reflect the stoichiometric ratio of each species in the equation.
Eg for a 2.0 M drop of NH 3there should be a 3.0 M increase in H2 and a 1.0 M increase in N2.
This is not clearly shown in our example on the right.
t3 - the temperature of the reaction vessel is cooled.
Cooling the reaction vessel decreases all rates, however the forward rate is reduced by a lesser amount than the rate of the reverse reaction thus allowing for a net forward movement by the system.
Note the gradual change of the concentration of all the species which is indicative of a temperature change.