The nuts and bolts of
chemical equations Part2
The reaction below represents the recipe of making water from hydrogen and oxygen gases.
It tells us that two molecules of hydrogen react with one molecule of oxygen to make 2 molecules of water.
![]()
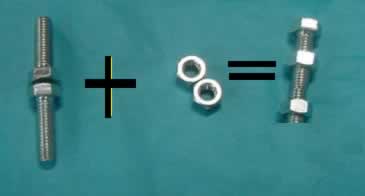
Using nuts and bolts assemble the reaction for the formation of water. The reaction pictured above is not balanced. Balanced means that all the bolts and nuts will be used up. In this example one bolt will remain. Try to add as many hydrogen, oxygen and water molecules as you need to balance this equation.
Using nuts and bolts assemble the reaction for the formation of aluminium oxide. The reaction pictured below is not balanced. In this example we need more aluminium atoms and oxygen molecules as well as units of aluminium oxide.
![]()
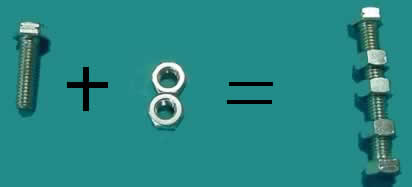
Using the nuts and bolts balance the equation
above.
Solution