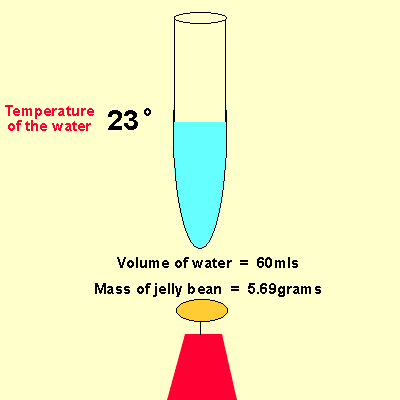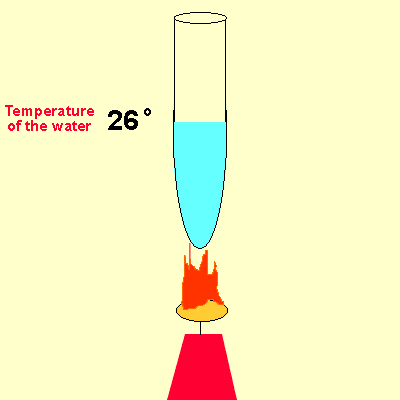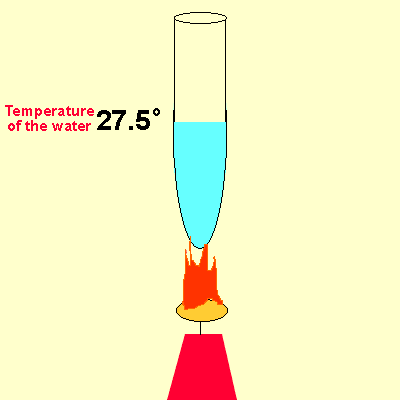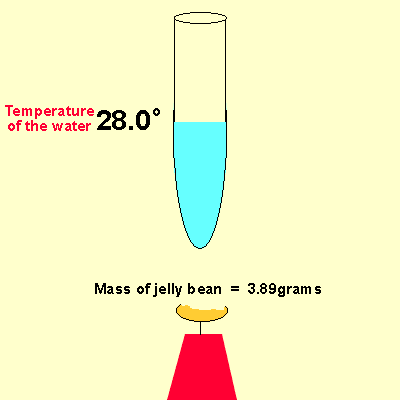
Solution
The reaction is an exothermic
reaction. Heat is given off.
The calibration factor of the calorimeter is given by the expression below.
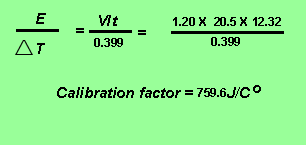
Obtain the amount of energy released from 0.02134 grams of ethene burnt in oxygen according the equation below
![]()
Calibration factor X change in temperature = 759.6 X 1.42 = 1.078KJ
The enthalpy change is energy/mol
SO!
Step 1) Mole of ethene added = 0.02134 /28 = 0.00076 mole
Step 2) Divide the amount of energy by the number of mole of ethene.
1.078 / 0.00076 = -1418.42Kj/mol
Note how the enthalpy change has a negative sign. This indicates an exothermic reaction (heat given out).
Click to hide the solution