Explaining Endothermic and Exothermic reactions
So breaking bonds requires an input of energy and making bonds causes energy to be given out to the surroundings.
View the animation on the right. It shows how two atoms behave as they break and form bonds between themselves.

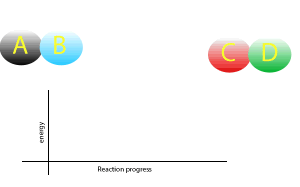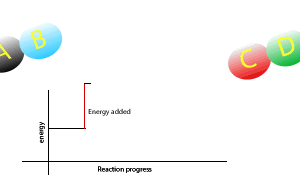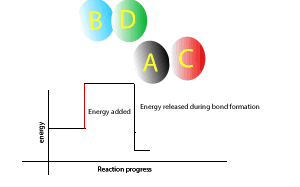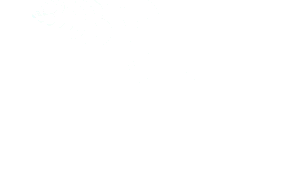
Now during a chemical reaction, atoms break the chemical bonds that exist between each other and rearrange to form new bonds between different atoms.
A chemical reaction that gives out more energy during bond formation than it uses in bond breaking is called an exothermic reaction.
During such reactions there is a net output of energy so the surroundings get hotter.
You will be familiar with these type of reactions as they are common in the kitchen. A good example of an exothermic reaction is when we burn gas to heat our food.
Clearly the amount of energy released during the formation of carbon dioxide and water is overwhelmingly higher than the energy needed to break apart the bonds between the atoms in the sugar.
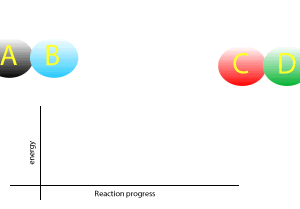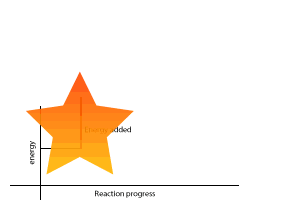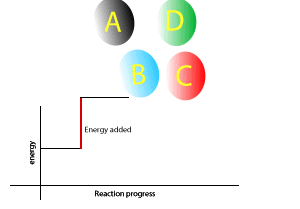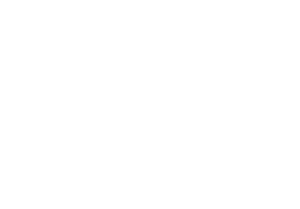
A chemical reaction that gives out less energy during bond formation than it uses in bond breaking is called an endothermic reaction.
During such reactions there is a net intake of energy so the surroundings become cooler.
Melting of ice, although it is not a chemical reaction, is an endothermic process where by ice absorbs energy from the surroundings to change state and thereby cooling the environment the ice is in.
1) The video on the left shows a normal cotton wool ball burning and a chemically treated cotton wool ball, called gun cotton, burning.
a) What types of reactions are shown in the video.
b) What can you say about the difference in the amount of energy given out during bond formation as compared to the amount of energy needed to break the bonds between the atoms of each cotton ball?
3) Methane reacts with oxygen to form water and carbon dioxide, as shown in the video above. The equation is shown below.
methane + oxygen --> carbon dioxide + water
Complete the energy profile, shown on the left, of the reaction between methane and oxygen.


a) Complete the energy diagram, shown on the left, as the ammonium nitrate dissolves in the water inside the cold pack.
b) Which one of the two terms below applies to the cold pack? Give a reason for your choice.
i. Absorbs heat from it's surroundings.
ii. Releases heat to its surroundings.
c) Explain why your skin feels cold when the cold pack is placed on the surface of an injury.