
The total energy of a system is made up of two types of energy, kinetic energy and potential energy. Kinetic energy comes from the movement of molecules in the gas and liquid phase or the vibration of particles in a solid.
Consider the reaction between atmospheric nitrogen and oxygen the equation is given below.
N2 + O2 => 2NO
ΔH= +181 kJ/mol
For every mol of nitrogen gas that reacts 181 kJ of energy is absorbed. This usually comes form lightning in the atmosphere.
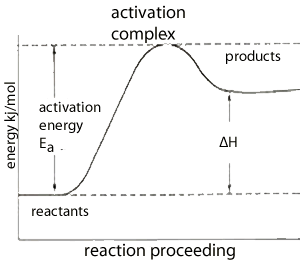
The energy profile on the left is typical of endothermic reactions which absorb energy from their surroundings.
Examples of endothermic reactions exist in our every day lives. The evaporation of sweat from our skin or the water in a car radiator are subtle examples of endothermic reactions. Cold packs for sports injuries are a more dynamic example of endothermic reactions absorbing heat from the surroundings.
An excellent demonstration of an endothermic reaction is the reaction of solid barium hydroxide with ammonium chloride.
Click to go to the demonstration.
"A system will try and move to the lowest possible energy or the greatest randomness or entropy".
With reference to the above comment discuss what happens when water goes from the liquid to the gas phase.
Solution
Why is energy needed from the surroundings?