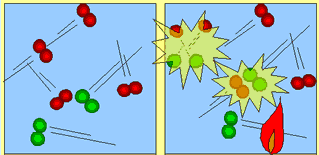
| Molecules move about and collide into each other. The force with which they collide is not enough to cause a chemical reaction. | As heat is added (activation energy) the molecules are forced to speed up. They collide more frequently and with enough force to break apart. Energy is released which fuels further collisions. |
|
The atoms now recombine to form a new product.
This new product has a greater hold on its atoms and takes a great
deal of force to actually break each molecule apart. Only in extreme
temperatures will the molecules collide with enough force as to
break apart.
|
For a reaction to occur, molecules must collide with
enough force so as to break apart. The individual atoms then recombine
to form a totally new product. This new product is usually very strong
in holding its atoms together. Some times a little heat is supplied to
get the molecules traveling at speeds high enough to cause energetic collisions.
This heat is called activation
energy. Petrol and oxygen for example can mix freely with
each other without reacting. When activation energy is provided in the
form of a spark the reaction proceeds with a huge release of energy and
force. The spark has caused molecules of oxygen and petrol to increase
in speed and collide with a force big enough to break the molecules apart.
This reaction releases heat and fuels more collisions
and the reaction soon becomes explosive.
Hydrogen and oxygen are used to fuel the Space Shuttle. They burn
to produce a great deal of heat. Air contains huge amounts of oxygen and
hydrogen but lighting a match in the open does not cause a huge explosion.
Explain WHY?
Candle wax does not burn but it will melt. However the wax vapour will ignite. Have a look at the 120kb movie and explain why wax vapour ignites while solid wax does not burn. Explain in terms of collisions.