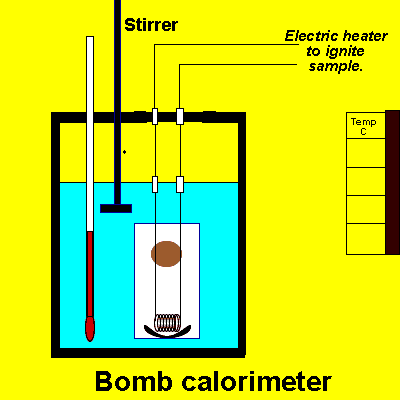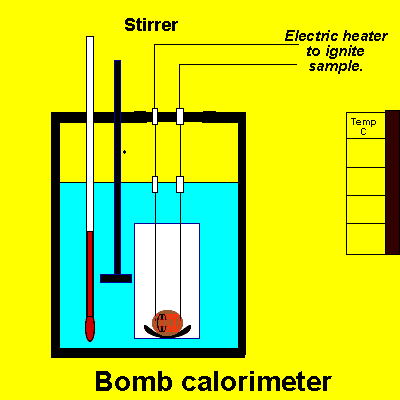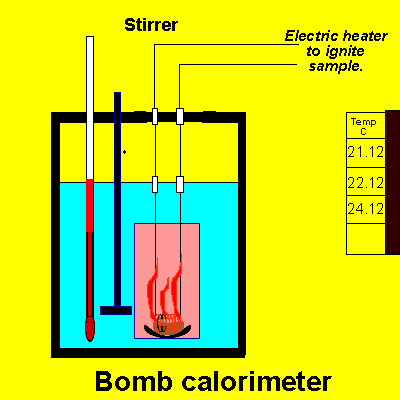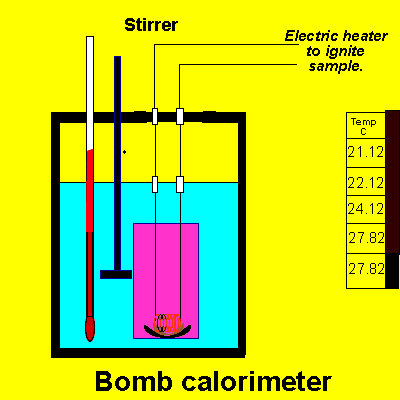
Solution
The reaction is an exothermic
reaction. Heat is given off.
To obtain the change in enthalpy for the reaction below
![]()
Step 1 find the number of joules
produced.
1kg of water increased in temperature by 6.7 degrees celsius
The amount of energy required to do this is given by the expression
heat capacity X mass of water X temperature change = 4.2 X 1000 X 6.7
= 28140 J or 28.4Kj.
Step 2) Mole of glucose added = 1.8 /180 = 0.01 mole
Step 3) Divide the amount of energy given out by the number of moles to get the enthalpy change.
28.40 / 0.01 = -2840Kj/mol
Note how the enthalpy change has a negative sign. This indicates an exothermic reaction (heat given out).
Click to hide the solution